Homopolysaccharides
The major homopolysaccharides are cellulose, chitin, starches (amylose and amylopectin), glycogen, and xylans.Cellulose, a linear glucose polymer linked β-1-4 is the predominant natural product in the biosphere. The allequatorial structure allows for extensive hydrogen bonding, whereas the axial, somewhat hydrophobic faces favor a nonaqueous environment. The resulting aggregates have a highly ordered, quasi-crytalline arrangement (Fig. 21); hence, the unusual stability exhibited by the molecule, exemplified in trees and wooden artifacts. The broad distributionandlowcost of cellulosehasmadeitamajor starting material for industrial development while the unmodifiedpolymer remains thebasis forwood, paper, andcotton.
Chitin, identical in linkage to cellulose but composed of N-acetylglucosamine instead of glucose, is the major structural component of insect and crustacean exoskeletons as well as a cell wall component of molds and fungi. The structural comments regarding cellulose also apply generally to chitin, especially in terms of stability. Less industrial development has been done with this polymer, in part because shrimp shells may present a more difficult starting material than trees.
Amylose and amylopectin are closely related, α-1-4- linked glucose
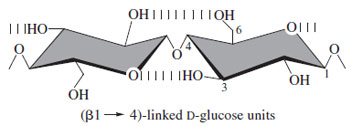 |
| Figure 21 The repeating unit of cellulose showing hydrogen bond interactions. The extended structure allows chains to stack via the relatively hydrophobic axial faces of the pyranose rings. |
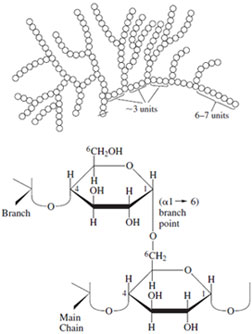 |
| Figure 22 Schematic structure for glycogen and details of a typical branch point. The same linkages are present in amylopectin, which has less frequent branches. |
Glycogen (Fig. 22), present in all higher animals, is closely related to amylopectin in that it has the same fundamental structure of a linear glucose chain with branches, and the same linkages. In this case, however, branches occur about every seventh residue, yielding a highly rebranched, tree-like envelope. This is essential for both packing in cells and for the rapid degradation of the polymer to provide critical metabolic intermediates. Glycogen serves as a primary energy reservoir in muscle and as a source of circulating glucose in the liver. It is of interest that the biosynthesis of glycogen initiates on a core protein (glycogenin), which may be cleaved from the polysaccharide subsequent to polymerization.
Xylans are a group of polymers based on a structure analogous to that of cellulose wherein xylose is the repeating unit. The simplest representative contains only D-xylose with β-1-4 linkages and is a common component of plant walls. Several heteropolysaccharides utilize the xylan backbone and have various other saccharides as branches. Xylans are often associated with cellulose in plant cell walls.




