O-Linked Glycoconjugates
O-linked glycoconjugates have substantial diversity in that the saccharide units may be covalently attached to serine, threonine, tyrosine, hydroxylysine, or hydroxyproline residues. In addition, the type of glycosyl substitution varies widely, from single sugars to extended polysaccharide chains. The following discussion highlights key features of these types but is not intended to provide full details.One major category of O-linked glycosylation is termed mucin type. This is characterized by linkage of the sugar (N-acetylgalactosamine in the alpha configuration) to serine or threonine hydroxyl groups (Fig. 27). There is no identifiable consensus amino acid sequence known which targets specific residues to be substituted. The saccharide units range from di- to intermediate size oligosaccharides (up to 10 sugars) and are very diverse. Additional sugars present include galactose, N-acetylglucosamine, L-fucose, and sialic acid; some of the saccharide units may be sulfated. Mannose is characteristically absent. These molecules are often found in epithelial secretions; the protein cores may be quite large with a single glycoprotein having an aggregate molecular weight of one million with a hundred or more saccharide units covalently attached.
Glycosylation of tyrosine residues is unusual but a key step in the biosynthesis of glycogen, the major storage glucan of liver and muscle. The core protein, glycogenin, is able to autoglucosylate and attaches a series of glucosyl residues to a single tyrosine in the protein. When the glucose chain has reached four (or more) units (all linked α-1-4), the resulting saccharide moiety is then recognized by glycogen synthase for continuation of glycogen formation. The final polysaccharide may have several thousand glucose residues.
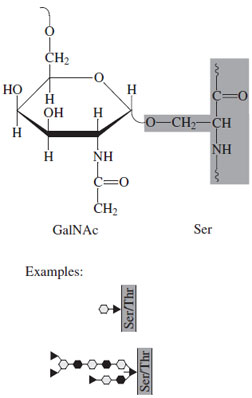 |
| Figure 27 Typical O-linked, mucin-type oligosaccharide. |
Glycosylation on hydroxyproline residues is rare but has been reported in both plants and fungi. Interestingly, this does not occur in collagen where hydroxyproline is a prominent residue.
An unusual form of O-glycosylation has recently been described wherein a single N-acetylglucosaminyl residue is attached to either a serine or threonine residue in target proteins. In contrast to other O-linked glycoproteins, the entities involved are cytosolic and not secreted. It has been suggested that this modification is reciprocal with phosphorylation, a common form of regulatory substitution.
A major class of O-linked glycoconjugates is the proteoglycans, key components of the extracellular matrix of animals. These glycoconjugates are distinguished by the linking sugar (D-xylose, attached to serine), a linear core saccharide (GaL-GaL-Xyl; Fig. 28), and continuation of the saccharide chain as a linear polysaccharide, which contains alternating residues of an amino sugar (N-acetylglucosamine or N-acetylgalactosamine) and a uronic acid (D-glucuronic or L-iduronic acid) (Fig. 29). The saccharides are generally sulfated (may include N-sulfation instead of N-acetylation of the glucosaminyl residues) giving rise to chains of considerable structural diversity. Examples include heparin, a natural anticoagulant, and the chondroitin sulfate proteoglycans. In the case of heparin, it has been established that the antithrombin activity resides in a specific pentasaccharide sequence within the structure with a defined pattern of sulfation and sugar components. This type of “information” is known to be present in other complex saccharides and broadens the function of these molecules beyond that of space occupancy and water and electrolyte management. It is interesting to note that the biosynthesis of the iduronosyl moiety in heparin and related heparan sulfate chains occurs at the polymer level by inversion of configuration at C-5 of already incorporated glucuronosyl residues. Extracellular proteoglycans such as those of the chondroitin and dermatan sulfate families are associated with organization of the fibrillar elements of connective tissues (primarily collagens), bone deposition and maintenance of tissue hydration.
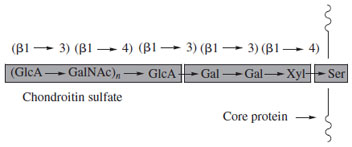 |
| Figure 28 Core saccharide of proteoglycans. |
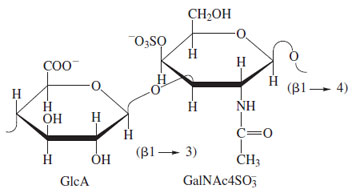 |
| Figure 29 Structure of the repeating unit of chondroitin 4- sulfate. Other glycosaminoglycan chains presents in proteoglycans include dermatan sulfate (L-iduronic acid replacing Dglucuronic acid), variants with sulfate in the 6-position, and the heparin–heparan sulfate family, which contains both uronic acids, glucosamine, and both N- and O-sulfate esters. |
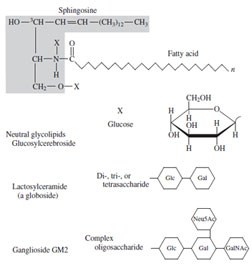 |
| Figure 30 Ceramide and glycosphingolipid structures. |




