Oligosaccharides
The ability of the anomeric hydroxyl group of a sugar to be substituted with another sugar (via one of its hydroxyl groups), and for this process to be iterated leads to formation of di- and higher saccharides. Because several hydroxyls are available for such linkages and the anomeric configuration can vary as well, the number of possible structures grows exponentially even for oligomers derived from the same sugar. This may explain, in part, why many biological recognition events involve saccharides as a ligand.Two disaccharides are widely distributed in nature: sucrose and lactose. Sucrose (α-D-glucopyranosyL-β-Dfructofuranoside) is the table sugar of commerce and a major industrial product (Fig. 18); primary sources are sugar cane and beets. This saccharide and its source material (cane molasses) serve as the basis for rum production; cruder precursors are important medium additives for the production of antibiotics. The sweetness of sucrose, and other simple sugars, is a major aspect of their commercial importance. The fact that sucrose is a nonreducing sugar contributes to its stability and market value.
Lactose (β-D-galactopyranosyL-1-4-D-glucopyranose) is the major sugar of milk (Fig. 19). It is of interest that the ability to utilize either sucrose or lactose as a source of calories is dependent on their enzymatic hydrolysis to the constituent monosaccharides in the intestine. About onethird of the oriental population lacks the requisite galactosidase and is thus lactose intolerant; this same problem occurs in some infants (developmentally related) and results in a “colicky” baby.
Other naturally occurring disaccharides of interest are maltose (α-D-glucopyranosyL-1-4-D-glucopyranose), an intermediate in the digestion of starches, and trehalose (α-D-glucopyranosyL-α-D-glucopyranoside), the major sugar of insect blood (Fig. 20).
Naturally occurring tri- and higher oligosaccharides (less than 10 sugar units) are rare. Raffinose (3), stachyose (4), and verbascose (5) are all assembled on a sucrose core with substituents originating on the glucose moiety.
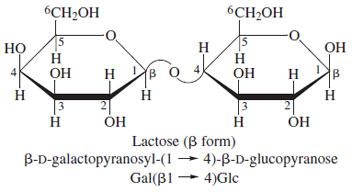 |
| Figure 19 Structure of lactose, the major sugar present in milk. |
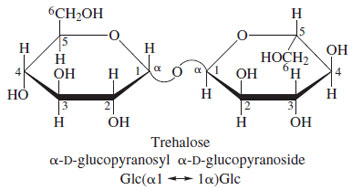 |
| Figure 20 Structure of trehalose, the predominant sugar present in the blood of insects. |




