Lateral Line System of Fish and Amphibians
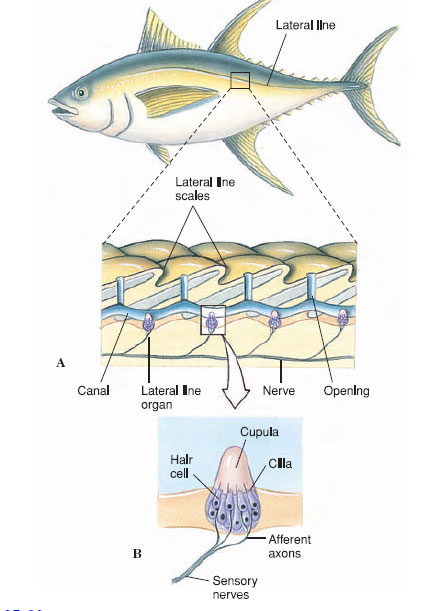 |
| Figure 35-22 Lateral line system. A, Lateral line of a bony fish with both exposed and hidden neuromasts. B, Structure of a neuromast (lateral line organ). |
A lateral line is a distant touch reception system for detecting wave vibrations and currents in water. Receptor cells, called neuromasts, are located on the body surface in aquatic amphibians and some fishes, but in many fishes they are located within canals running beneath the epidermis; these canals open at intervals to the surface (Figure 35-22). Each neuromast is a collection of hair cells with sensory endings, or cilia, embedded in a gelatinous, wedge-shaped mass, the cupula. The cupula projects into the center of the lateral line canal so that it bends in response to any disturbance of water on the body surface. The lateral line system is one of the principal sensory systems that guide fishes in their movements and in location of predators, prey, and social partners.
Hearing
An ear is a specialized receptor for detecting sound waves in the surrounding environment. Because sound communication and reception are integral to the lives of terrestrial vertebrates, we may be surprised to discover that most invertebrates inhabit a silent world. Only certain arthropod groups—crustaceans, spiders, and insects— have developed true soundreceptor organs. Even among insects, only locusts, cicadas, crickets, grasshoppers, and most moths possess ears, and these are of simple design: a pair of air pockets, each enclosed by a tympanic membrane that passes sound vibrations to sensory cells. Despite their spartan construction, insect ears are beautifully designed to detect the sound of a potential mate, a rival male, or a predator.
Especially interesting are the ultrasonic detectors of certain nocturnal moths. These have evolved specifically to detect approaching bats and thus lessen the moth’s chance of becoming a bat’s evening meal (echolocation in bats is described on). Each moth ear possesses just two sensory receptors, known as A1 and A2 (Figure 35-23). The A1 receptor will respond to ultrasonic cries of a bat that is still too far away to detect the moth. As the bat approaches and its cries increase in intensity, the receptor fires more rapidly, informing the moth that the bat is coming nearer. Since the moth has two ears, its nervous system can determine the bat’s position by comparing firing rates from the two ears. The moth’s strategy is to fly away before the bat detects it. But if the bat continues its approach, the second (A2) receptor in each ear, which responds only to high-intensity sounds, will fire. The moth responds immediately with an evasive maneuver, usually making a power dive to a bush or the ground where it is safe because the bat cannot distinguish the moth’s echo from those of its surroundings.
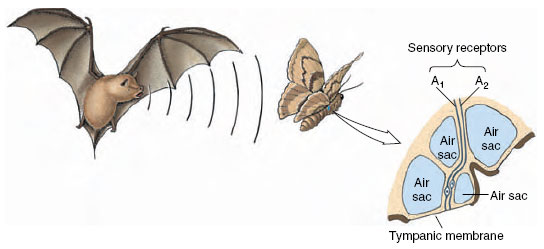 |
| Figure 35-23 Ear of a moth used to detect approaching bats. See text for explanation. |
In its evolution, the vertebrate ear originated as a balance organ, the labyrinth. In all jawed vertebrates, from fishes to mammals, the labyrinth has a similar structure, consisting of two small chambers called the saccule and the utricle, and three semicircular canals (Figure 35-24). In fish the base of the saccule is extended into a tiny pocket (the lagena) that, during the evolution of the vertebrates, developed into the hearing receptor of tetrapods. With continued elaboration and elongation in the birds and mammals, the fingerlike lagena was modified go form the cochlea.
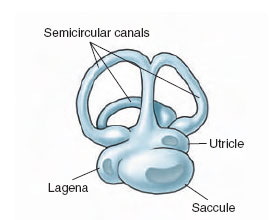 |
| Figure 35-24 Vestibular apparatus of a teleost fish, containing three semicircular canals, which respond to angular acceleration; two balance organs (utricle and saccule), which are static receptors that signal the fish’s position in relation to gravity; and a small chamber, the lagena, which is specialized for sound reception. |
The human ear (Figure 35-25) is representative of mammalian ears. The outer, or external, ear collects sound waves and funnels them through the auditory canal to the eardrum or tympanic membrane lying next to the middle ear. The middle ear is an airfilled chamber containing a remarkable chain of three tiny bones, or ossicles, known as the malleus (hammer), incus (anvil), and stapes (stirrup), named because of their fancied resemblance to these objects. These bones conduct sound waves across the middle ear (Figure 35-25B). The bridge of bones is so arranged that the force of sound waves pushing against the tympanic membrane is amplified as much as 90 times where the stapes contacts the oval window of the inner ear. Muscles attached to the middle ear bones contract when the ear receives very loud noises, providing the inner ear some protection from damage. The middle ear connects with the pharynx by means of the eustachian tube, which permits pressure equalization on both sides of the tympanic membrane.
The origin of the three tiny bones of the mammalian middle ear—the malleus, incus, and stapes—is one of the most extraordinary and well-documented transitions in vertebrate evolution. Amphibians, reptiles,and birds have a single rodlike ear ossicle, the stapes (also called the columella),which originated as a jaw support (the hyomandibular) as seen in fishes (see Figure 25-16,). With evolution of the earliest tetrapods, the braincase became firmly sutured to the skull, and the hyomandibular, no longer needed to brace the jaw,became converted into the stapes. In a similar way, the two additional ear ossicles of the mammalian middle ear—the malleus and incus— originated from parts of the jaw of early vertebrates. The quadrate bone of the reptilian upper jaw became the incus, and the articular bone of the lower jaw became the malleus.Homology of reptilian jaw bones to mammalian ear bones is clearly documented in the fossil record and in embryological development of mammals.
Within the inner ear is the organ of hearing, the cochlea (Gr. cochlea, snail’s shell), which is coiled in mammals, making two and one half turns in humans (Figure 35-25B). The cochlea is divided longitudinally into three tubular canals running parallel with one another. This relationship is indicated in Figure 35-26. These canals become progressively smaller from the base of the cochlea to the apex. One of these canals is called the vestibular canal; its base is closed by the oval window. The tympanic canal, which is in communication with the vestibular canal at the tip of the cochlea, has its base closed by the round window. Between these two canals is the cochlear duct, which contains the organ of Corti, the actual sensory apparatus (Figure 35-25C). Within the organ of Corti are fine rows of hair cells that run lengthwise from the base to the tip of the cochlea. At least 24,000 hair cells are present in the human ear. The 80 to 100 “hairs” on each cell are actually microvilli and a single large cilium, which project into the endolymph of the cochlear canal. Each cell is connected with neurons of the auditory nerve. The hair cells rest on the basilar membrane, which separates the tympanic canal and cochlear duct, and they are covered by the tectorial membrane, lying directly above them (Figure 35-25D).
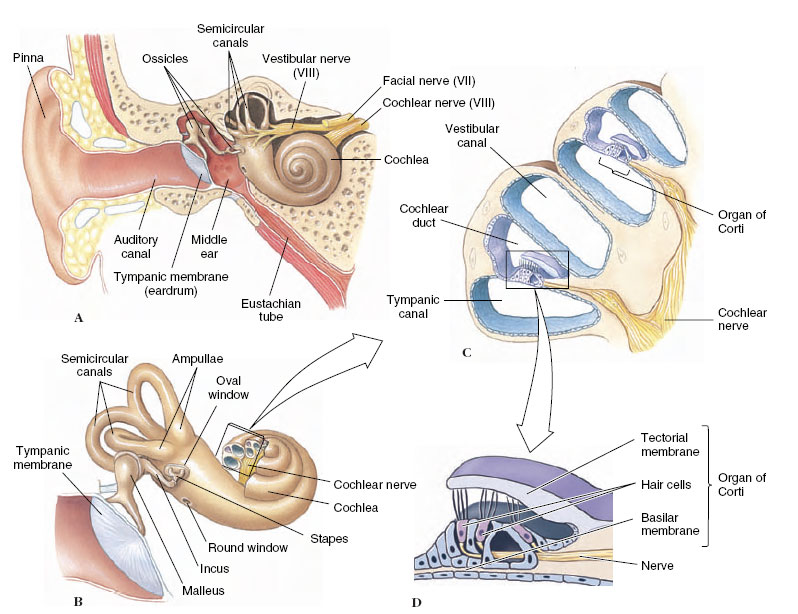 |
| Figure 35-25 Human ear. A, Longitudinal section showing external, middle, and inner ear. B, Enlargement of middle ear and inner ear. The cochlea of the inner ear has been opened to show the arrangement of canals within. C, Enlarged cross section of cochlea showing the organ of Corti. D, Detail of ultrastructure of the organ of Corti. |
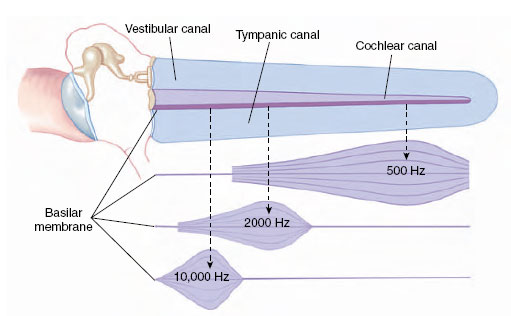 |
| Figure 35-26 Frequency localization in the cochlea of the mammalian ear as it would appear with the cochlea stretched out. Sound waves transmitted to the oval window produce vibration waves that travel down the basilar membrane. High-frequency vibrations cause the membrane to resonate near the oval window. Lowfrequency tones travel farther down the basilar membrane. |
When a sound wave strikes the ear, its energy is transmitted through the ossicles of the middle ear to the oval window, which oscillates back and forth, driving the fluid of the vestibular and tympanic canals before it. Because these fluids are noncompressible, an inward movement of the oval window produces a corresponding outward movement of the round window. The fluid oscillations also cause the basilar membrane with its hair cells to vibrate simultaneously.
According to the place hypothesis of pitch discrimination formulated by Georg von Békésy, different areas of the basilar membrane respond to different frequencies; for every sound frequency, there is a specific “place” on the basilar membrane where the hair cells respond to that frequency. Initial displacement of the basilar membrane starts a wave traveling down the membrane, much as flipping a rope at one end starts a wave moving down the rope (Figure 35-27). The displacement wave increases in amplitude as it moves from the oval window toward the apex of the cochlea, reaching a maximum at the region of the basilar membrane where the natural frequency of the membrane corresponds to the sound frequency. Here, the membrane vibrates with such ease that the energy of the traveling wave is completely dissipated. Hair cells within the organ of Corti in that region are stimulated and the impulses conveyed to the fibers of the auditory nerve. Isolated hair cells have been shown to respond to a particular band of frequencies depending on their location within the cochlea. Thus, impulses that are carried by certain fibers of the auditory nerve are interpreted by the hearing center as particular tones. The loudness of a tone depends on the number of hair cells stimulated, whereas the timbre, or quality, of a tone is produced by the pattern of hair cells stimulated by sympathetic vibration. This latter characteristic of tone enables us to distinguish between different human voices and different musical instruments, although the notes in each case may be of the same pitch and loudness.
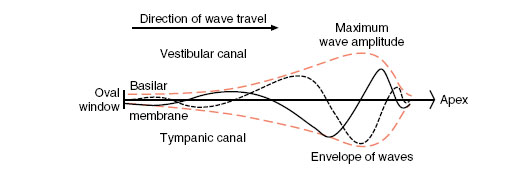 |
| Figure 35-27 Traveling waves along the basilar membrane. The oval window is at left, and the cochlear apex at right. The two wave formations (solid and dashed lines) occur at separate instants of time. The curves in color represent the extreme displacements of the membrane by traveling waves. |
Most recent auditory research has focused on a more active role for the hair cells within the organ of Corti. Experiments have demonstrated that outer hair cells may respond to sound waves by changing their length and thus mechanically altering the position of the basilar and tectorial membranes. Although a function of such movements is not yet established, it has been suggested that this active response of these receptor cells in the organ of Corti might increase both the sensitivity and selectivity of hearing.




