Muscular Movement
Muscular Movement
Contractile tissue is most highly developed in muscle cells called fibers. Although muscle fibers themselves can do work only by contraction and cannot actively lengthen, they can be arranged in so many different configurations and combinations that almost any movement is possible.
Types of Vertebrate Muscle
Vertebrate muscle is broadly classified on the basis of the appearance of muscle cells (fibers) when viewed with a light microscope. Skeletal muscle appears transversely striped (striated), with alternating dark and light bands (Figure 31-13). Cardiac muscle also possesses striations like skeletal muscle but is uninucleate and with branching cells. A third type of vertebrate muscle is smooth (or visceral) muscle which lacks the characteristic alternating bands of the striated type.
Skeletal muscle is typically organized into sturdy, compact bundles or bands (Figure 31-13A). It is called skeletal muscle because it is attached to skeletal elements and is responsible for movements of the trunk, appendages, respiratory organs, eyes, mouthparts, and other structure. Skeletal muscle fibers are extremely long, cylindrical, multinucleate cells that may reach from one end of the muscle to the other. They are packed into bundles called fascicles (L. fasciculus, small bundle), which are enclosed by tough connective tissue. The fascicles are in turn grouped into a discrete muscle surrounded by a thick connective tissue layer. Most skeletal muscles taper at their ends, where they connect to bones by tendons. Other muscles, such as the ventral abdominal muscles, are flattened sheets.
In most fishes, amphibians, and to some extent lizards and snakes, there is a segmented organization of muscles alternating with the vertebrae. The skeletal muscles of other vertebrates, by splitting, fusion, and shifting, have developed into specialized muscles best suited for manipulating jointed appendages that have evolved for locomotion on land. Skeletal muscle contracts powerfully and quickly but fatigues more rapidly than does smooth muscle. Skeletal muscle is sometimes called voluntary muscle because it is stimulated by motor fibers and is under conscious cerebral control.
Smooth muscle lacks the striations typical of skeletal muscle (Figure 31-13B). The cells are long, tapering strands, each containing a single nucleus. Smooth muscle cells are organized into sheets of muscle circling the walls of the alimentary canal, blood vessels, respiratory passages, and urinary and genital ducts. Smooth muscle is typically slow acting and can maintain prolonged contractions with very little energy expenditure. It is under the control of the autonomic nervous system; thus, unlike skeletal muscle, its contractions are involuntary and unconscious. The principal functions of smooth muscles are to push material in a tube, such as the intestine, along its way by active contractions or to regulate the diameter of a tube, such as a blood vessel, by sustained contraction.
Cardiac muscle, the seemingly tireless muscle of the vertebrate heart, combines certain characteristics of both skeletal and smooth muscle (Figure 31-13C). It is fast acting and striated like skeletal muscle, but contraction is under involuntary autonomic control like smooth muscle. Actually the autonomic nerves serving the heart can only speed up or slow down the rate of contraction; the heartbeat originates within specialized cardiac muscle, and the heart continues to beat even after all autonomic nerves are severed (heart excitation is described on). Cardiac muscle is composed of closely opposed, but separate, uninucleate cell fibers.
Types of Invertebrate Muscle
Smooth and striated muscles are also characteristic of invertebrate animals, but there are many variations of both types and even instances in which structural and functional features of vertebrate smooth and striated muscle are combined. Striated muscle appears in invertebrate groups as diverse as cnidarians and arthropods. The thickest muscle fibers known, approximately 3 mm in diameter and 6 cm long, are those of giant barnacles and of Alaska king crabs living along the Pacific coast of North America. Such large muscle cells lend themselves well to physiological studies and are understandably popular with muscle physiologists.
In the limited space available to treat the great diversity of muscle structure and function in the invertebrate assemblage, we have selected for discussion two functional extremes: the specialized adductor muscles of molluscs and the fast flight muscles of insects.
Bivalve molluscan muscles contain fibers of two types. One kind is striated muscle that can contract rapidly, enabling the bivalve to snap shut its valves when disturbed. Scallops use these “fast” muscle fibers to swim in their awkward manner (see Figure 16-24B,). The second muscle type is smooth muscle, capable of slow, long-lasting contractions. Using these fibers, a bivalve can keep its valves tightly shut for hours or even days. Such adductor muscles use little metabolic energy and receive remarkably few nerve impulses to maintain the activated state. The contracted state has been likened to a “catch mechanism” involving some kind of stable cross-linkage between contractile proteins within the fiber. However, despite considerable research, there is still much uncertainty about how this adductor mechanism works.
Insect flight muscles are virtually the functional antithesis of the slow, holding muscles of bivalves. The wings of some small flies operate at frequencies greater than 1000 beats per second. The so-called fibrillar muscle, which contracts at these frequencies— far greater than even the most active of vertebrate muscles—shows unique characteristics. It has very limited extensibility; that is, the wing leverage system is arranged so that the muscles shorten only slightly during each downbeat of the wings. Furthermore, muscles and wings operate as a rapidly oscillating system in an elastic thorax (see Figure 20-12). Since the muscles rebound elastically and are activated by stretch during flight, they receive impulses only periodically rather than one impulse per contraction; one reinforcement impulse for every 20 or 30 contractions is enough to keep the system active. Insect flight muscles are described in more detail in Terrestrial Mandibulates.
Structure of Striated Muscle
As mentioned earlier, striated muscle is so named because of periodic bands, plainly visible under the light microscope, that pass across the widths of muscle cells. Each cell, or fiber, is a multinucleated tube containing numerous myofibrils, packed together and invested by the cell membrane, the sarcolemma (Figure 31-14). The myofibril contains two types of myofilaments: thick filaments composed of the protein myosin, and thin filaments, composed of the protein actin. These are the actual contractile proteins of the muscle. Thin filaments are held together by a dense structure called the Z line. The functional unit of the myofibril, the sarcomere, extends between successive Z lines. These anatomical relationships are diagramed in Figure 31-14.
Human muscle tissue develops before birth, and a newborn child’s complement of skeletal muscle fibers is all that he or she will ever have. But while an adult male weight lifter and a young boy have a similar number of muscle fibers, the weight lifter may be several times the boy’s strength because repeated high-intensity, shortduration exercise has induced the synthesis of additional actin and myosin filaments. Each fiber has hypertrophied, becoming larger and stronger. Endurance exercise such as long-distance running produces a very different response. Fibers do not become greatly stronger but develop more mitochondria and myoglobin and become adapted for a high rate of oxidative phosphorylation. These changes, together with the development of more capillaries serving the fibers, lead to increased capacity for long-duration activity.
Each thick filament is made up of myosin molecules packed together in an elongate bundle (Figure 31-15). Each myosin molecule is composed of two polypeptide chains, each having a club-shaped head. Lined up as they are in a bundle to form a thick filament, the double heads of each myosin molecule face outward from the center of the filament. These heads act as molecular cross bridges that interact with the thin filaments during contraction.
Thin filaments are more complex because they are composed of three different proteins. The backbone of the thin filament is a double strand of the protein actin, twisted into a double helix. Surrounding the actin filament are two thin strands of another protein, tropomyosin, that lie near the grooves between the actin strands. Each tropomyosin strand is itself a double helix as shown in Figure 31-15C.
The third protein of the thin filament is troponin, a complex of three globular proteins located at intervals along the filament. Troponin is a calcium-dependent switch that acts as the control point in the contraction process.
Sliding Filament Model of Muscle Contraction
In the 1950s the English physiologists A. F. Huxley and H. E. Huxley independently proposed the sliding filament model to explain striated muscle contraction. According to this model, the thick and thin filaments become linked together by molecular cross bridges, which act as levers to pull the filaments past each other. During contraction, cross bridges on the thick filaments swing rapidly back and forth, alternately attaching to and releasing from special receptor sites on the thin filaments, and drawing thin filaments past thick in a kind of ratchet action. As contraction continues, the Z lines are pulled closer together (Fig-ure 31-16). Thus the sarcomere shortens. Because all sarcomere units shorten together, the muscle contracts. Relaxation is a passive process. When cross bridges between the thick and thin filaments release, the sarcomeres are free to lengthen. This requires some force, which is usually supplied by antagonistic muscles or the force of gravity.
Control of Contraction
Muscle contracts in response to nerve stimulation. If the nerve supply to a muscle is severed, the muscle atrophies, or wastes away. Skeletal muscle fibers are innervated by motor neurons whose cell bodies are located in the spinal cord. Each cell body gives rise to a motor axon that leaves the spinal cord to travel by way of a peripheral nerve trunk to a muscle where it branches repeatedly into many terminal branches. Each terminal branch innervates a single muscle fiber. Depending on the type of muscle, a single motor axon may innervate as few as three or four muscle fibers (where very precise control is needed, such as the muscles that control eye movement) or as many as 2000 muscle fibers (where precise control is not required, such as large leg muscles). The motor neuron and all muscle fibers it innervates is called a motor unit. The motor unit is the functional unit of skeletal muscle. When a motor neuron fires, the action potential passes to all fibers of the motor unit and each is stimulated to contract simultaneously. Total force exerted by a muscle depends on the number of motor units activated. Precise control of movement is achieved by varying the number of motor units activated at any one time. A smooth and steady increase in muscle tension is produced by increasing the number of motor units brought into play; this is called motor unit recruitment.
The Myoneural Junction
The place where a motor axon terminates on a muscle fiber is called the myoneural junction (Figure 31-17). At the junction is a tiny gap, or synaptic cleft, that thinly separates a nerve fiber and muscle fiber. In the vicinity of the junction, the neuron stores a chemical, acetylcholine, in minute vesicles known as synaptic vesicles. Acetylcholine is released when a nerve impulse reaches a synapse. This substance is a chemical mediator that diffuses across the narrow junction and acts on the muscle fiber membrane to generate an electrical depolarization. The depolarization spreads rapidly through the muscle fiber, causing it to contract. Thus the synapse is a special chemical bridge that couples together the electrical activities of nerve and muscle fibers.
Built into vertebrate skeletal muscle is an elaborate conduction system that serves to carry the depolarization from the myoneural junction to the densely packed filaments within the fiber. Along the surface of the sarcolemma are numerous invaginations that project as a system of tubules into the muscle fiber. This is called the T-system (Figure 31-17). The T-system is continuous with the sarcoplasmic reticulum, a system of fluid-filled channels that runs parallel to the myofilaments. The system is ideally arranged for speeding the electrical depolarization from the myoneural junction to the myofilaments within the fiber.
Excitation-Contraction Coupling
How does electrical depolarization activate the contractile machinery? In resting, unstimulated muscle, shortening does not occur because thin tropomyosin strands surrounding the actin myofilaments lie in a position that prevents the myosin heads from attaching to actin. When muscle is stimulated and the electrical depolarization arrives at the sarcoplasmic reticulum surrounding the fibrils, calcium ions are released (Figure 31-17). Some calcium binds to the control protein troponin. Troponin immediately undergoes changes in shape that allow tropomyosin to move out of its blocking position, exposing active sites on the actin myofilaments. The myosin heads then bind to these sites, forming cross bridges between adjacent thick and thin myofilaments. This sets in motion an attach-pull-release cycle that occurs in a series of steps as shown in Figure 31-18. Release of bond energy from ATP activates the myosin head, which swings 45 degrees, at the same time releasing a molecule of ADP. This is the power stroke that pulls the actin filament a distance of about 10 nm, and it comes to an end when another ATP molecule binds to the myosin head, inactivating the site. Thus each cycle requires expenditure of energy in the form of ATP (Figure 31-18).
Shortening will continue as long as nerve impulses arrive at the myoneural junction and free calcium remains available around the myofilaments. The attach-pull-release cycle can repeat again and again, 50 to 100 times per second, pulling thick and thin filaments past each other. While the distance each sarcomere can shorten is very small, this distance is multiplied by the thousands of sarcomeres lying end to end in a muscle fiber. Consequently, a strongly contracting muscle may shorten by as much as one-third its resting length.
When stimulation stops, calcium is quickly pumped back into the sarcoplasmic reticulum. Troponin resumes its original configuration; tropomyosin moves back into its blocking position on actin, and the muscle relaxes.
Energy for Contraction
Muscle contraction requires large amounts of energy. ATP is the immediate source of energy, but the amount present will sustain contraction for only a second or two. Muscle cells immediately call on the second level of energy reserve, creatine phosphate. Creatine phosphate is a high-energy phosphate compound that stores bond energy during periods of rest. As ADP is produced during contraction, creatine phosphate releases its stored bond energy to convert ADP to ATP. This reaction can be summarized as:
Within a few seconds—perhaps as long as 30 seconds depending on the rapidity of muscle contraction—the reserves of creatine phosphate are depleted. The contracting muscle now must be fueled from its third and largest store of energy, glycogen. Glycogen is a polysaccharide chain of glucose molecules stored in both liver and muscle. Muscle has by far the larger store—some threefourths of all the glycogen in the body is stored in muscle. As a supply of energy for contraction, glycogen has three important advantages: it is relatively abundant, it can be mobilized quickly, and it can provide energy under anoxic conditions. As soon as the muscle’s store of creatine phosphate declines, enzymes break down glycogen, converting it into glucose-6- phosphate, the first stage of glycolysis that leads into mitochondrial respiration and the generation of ATP.
If muscular contraction is not too vigorous or too prolonged, the glucose released from glycogen can be completely oxidized to carbon dioxide and water by aerobic metabolism. During prolonged or heavy exercise, however, blood flow to the muscles, although greatly increased above the resting level, cannot supply oxygen to the mitochondria rapidly enough to complete oxidation of glucose. The contractile machinery then receives its energy largely by anaerobic glycolysis, a process that does not require oxygen. The ability to take advantage of this anaerobic pathway, although not nearly as efficient as the aerobic one, is of great importance; without it, all forms of heavy muscular exertion would be impossible.
During anaerobic glycolysis, glucose is degraded to lactic acid with release of energy. This is used to resynthesize creatine phosphate, which in turn passes the energy to ADP for the resynthesis of ATP. Lactic acid accumulates in the muscle and diffuses rapidly into the general circulation. If muscular exertion continues, the buildup of lactic acid causes enzyme inhibition and fatigue. Thus the anaerobic pathway is a self-limiting one, since continued heavy exertion leads to exhaustion. The muscles incur an oxygen debt because accumulated lactic acid must be oxidized by extra oxygen. After a period of exertion, oxygen consumption remains elevated until all of the lactic acid has been oxidized or resynthesized to glycogen.
Contractile tissue is most highly developed in muscle cells called fibers. Although muscle fibers themselves can do work only by contraction and cannot actively lengthen, they can be arranged in so many different configurations and combinations that almost any movement is possible.
Types of Vertebrate Muscle
Vertebrate muscle is broadly classified on the basis of the appearance of muscle cells (fibers) when viewed with a light microscope. Skeletal muscle appears transversely striped (striated), with alternating dark and light bands (Figure 31-13). Cardiac muscle also possesses striations like skeletal muscle but is uninucleate and with branching cells. A third type of vertebrate muscle is smooth (or visceral) muscle which lacks the characteristic alternating bands of the striated type.
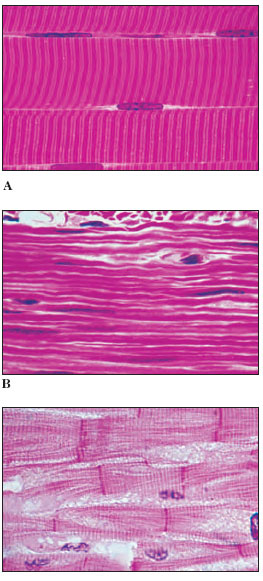 |
| Figure 31-13 Photomicrographs of types of vertebrate muscle. A, Skeletal muscle (human) showing several striated fibers (cells) lying side by side. Note the peripheral nuclei. B, Smooth muscle (human) showing absence of striations. Note elongate nuclei in the long fibers. C, Cardiac muscle (monkey). Note the vertical bars, called intercalated discs, joining separate fibers end to end. |
Skeletal muscle is typically organized into sturdy, compact bundles or bands (Figure 31-13A). It is called skeletal muscle because it is attached to skeletal elements and is responsible for movements of the trunk, appendages, respiratory organs, eyes, mouthparts, and other structure. Skeletal muscle fibers are extremely long, cylindrical, multinucleate cells that may reach from one end of the muscle to the other. They are packed into bundles called fascicles (L. fasciculus, small bundle), which are enclosed by tough connective tissue. The fascicles are in turn grouped into a discrete muscle surrounded by a thick connective tissue layer. Most skeletal muscles taper at their ends, where they connect to bones by tendons. Other muscles, such as the ventral abdominal muscles, are flattened sheets.
In most fishes, amphibians, and to some extent lizards and snakes, there is a segmented organization of muscles alternating with the vertebrae. The skeletal muscles of other vertebrates, by splitting, fusion, and shifting, have developed into specialized muscles best suited for manipulating jointed appendages that have evolved for locomotion on land. Skeletal muscle contracts powerfully and quickly but fatigues more rapidly than does smooth muscle. Skeletal muscle is sometimes called voluntary muscle because it is stimulated by motor fibers and is under conscious cerebral control.
Smooth muscle lacks the striations typical of skeletal muscle (Figure 31-13B). The cells are long, tapering strands, each containing a single nucleus. Smooth muscle cells are organized into sheets of muscle circling the walls of the alimentary canal, blood vessels, respiratory passages, and urinary and genital ducts. Smooth muscle is typically slow acting and can maintain prolonged contractions with very little energy expenditure. It is under the control of the autonomic nervous system; thus, unlike skeletal muscle, its contractions are involuntary and unconscious. The principal functions of smooth muscles are to push material in a tube, such as the intestine, along its way by active contractions or to regulate the diameter of a tube, such as a blood vessel, by sustained contraction.
Cardiac muscle, the seemingly tireless muscle of the vertebrate heart, combines certain characteristics of both skeletal and smooth muscle (Figure 31-13C). It is fast acting and striated like skeletal muscle, but contraction is under involuntary autonomic control like smooth muscle. Actually the autonomic nerves serving the heart can only speed up or slow down the rate of contraction; the heartbeat originates within specialized cardiac muscle, and the heart continues to beat even after all autonomic nerves are severed (heart excitation is described on). Cardiac muscle is composed of closely opposed, but separate, uninucleate cell fibers.
Types of Invertebrate Muscle
Smooth and striated muscles are also characteristic of invertebrate animals, but there are many variations of both types and even instances in which structural and functional features of vertebrate smooth and striated muscle are combined. Striated muscle appears in invertebrate groups as diverse as cnidarians and arthropods. The thickest muscle fibers known, approximately 3 mm in diameter and 6 cm long, are those of giant barnacles and of Alaska king crabs living along the Pacific coast of North America. Such large muscle cells lend themselves well to physiological studies and are understandably popular with muscle physiologists.
In the limited space available to treat the great diversity of muscle structure and function in the invertebrate assemblage, we have selected for discussion two functional extremes: the specialized adductor muscles of molluscs and the fast flight muscles of insects.
Bivalve molluscan muscles contain fibers of two types. One kind is striated muscle that can contract rapidly, enabling the bivalve to snap shut its valves when disturbed. Scallops use these “fast” muscle fibers to swim in their awkward manner (see Figure 16-24B,). The second muscle type is smooth muscle, capable of slow, long-lasting contractions. Using these fibers, a bivalve can keep its valves tightly shut for hours or even days. Such adductor muscles use little metabolic energy and receive remarkably few nerve impulses to maintain the activated state. The contracted state has been likened to a “catch mechanism” involving some kind of stable cross-linkage between contractile proteins within the fiber. However, despite considerable research, there is still much uncertainty about how this adductor mechanism works.
Insect flight muscles are virtually the functional antithesis of the slow, holding muscles of bivalves. The wings of some small flies operate at frequencies greater than 1000 beats per second. The so-called fibrillar muscle, which contracts at these frequencies— far greater than even the most active of vertebrate muscles—shows unique characteristics. It has very limited extensibility; that is, the wing leverage system is arranged so that the muscles shorten only slightly during each downbeat of the wings. Furthermore, muscles and wings operate as a rapidly oscillating system in an elastic thorax (see Figure 20-12). Since the muscles rebound elastically and are activated by stretch during flight, they receive impulses only periodically rather than one impulse per contraction; one reinforcement impulse for every 20 or 30 contractions is enough to keep the system active. Insect flight muscles are described in more detail in Terrestrial Mandibulates.
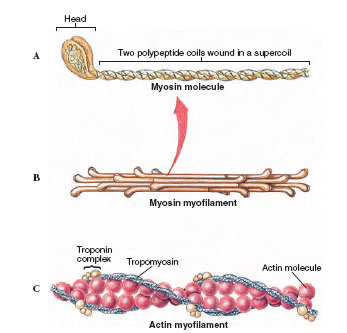 |
| Figure 31-15 Molecular structure of thick and thin myofilaments of skeletal muscle. A, The myosin molecule is composed of two polypeptides coiled together and expanded at their ends into a globular head. B, The thick myofilament is composed of a bundle of myosin molecules with the globular heads extended outward. C, The thin myofilament consists of a double strand of actin surrounded by two tropomyosin strands. A globular protein complex, troponin, occurs in pairs at every seventh actin unit. Troponin is a calcium-dependent switch that controls the interaction between actin and myosin. |
Structure of Striated Muscle
As mentioned earlier, striated muscle is so named because of periodic bands, plainly visible under the light microscope, that pass across the widths of muscle cells. Each cell, or fiber, is a multinucleated tube containing numerous myofibrils, packed together and invested by the cell membrane, the sarcolemma (Figure 31-14). The myofibril contains two types of myofilaments: thick filaments composed of the protein myosin, and thin filaments, composed of the protein actin. These are the actual contractile proteins of the muscle. Thin filaments are held together by a dense structure called the Z line. The functional unit of the myofibril, the sarcomere, extends between successive Z lines. These anatomical relationships are diagramed in Figure 31-14.
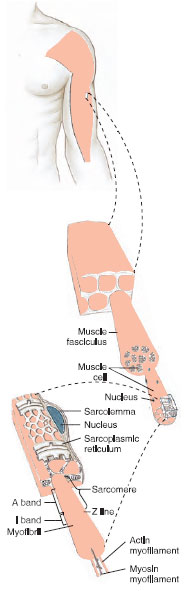 |
| Figure 31-14 Organization of skeletal muscle from gross to molecular level. A skeletal muscle (top) is composed of thousands of multinucleated muscle fibers (center), each containing thousands of myofibrils (bottom). Each myofibril contains numerous thick (myosin) and thin (actin) filaments that interact to slide past each other during contraction to shorten the muscle. The sarcoplasmic reticulum is a network of tubules surrounding the myofibrils that serves as a communication system for carrying a depolarization to the filaments within the muscle fiber. |
Human muscle tissue develops before birth, and a newborn child’s complement of skeletal muscle fibers is all that he or she will ever have. But while an adult male weight lifter and a young boy have a similar number of muscle fibers, the weight lifter may be several times the boy’s strength because repeated high-intensity, shortduration exercise has induced the synthesis of additional actin and myosin filaments. Each fiber has hypertrophied, becoming larger and stronger. Endurance exercise such as long-distance running produces a very different response. Fibers do not become greatly stronger but develop more mitochondria and myoglobin and become adapted for a high rate of oxidative phosphorylation. These changes, together with the development of more capillaries serving the fibers, lead to increased capacity for long-duration activity.
Each thick filament is made up of myosin molecules packed together in an elongate bundle (Figure 31-15). Each myosin molecule is composed of two polypeptide chains, each having a club-shaped head. Lined up as they are in a bundle to form a thick filament, the double heads of each myosin molecule face outward from the center of the filament. These heads act as molecular cross bridges that interact with the thin filaments during contraction.
Thin filaments are more complex because they are composed of three different proteins. The backbone of the thin filament is a double strand of the protein actin, twisted into a double helix. Surrounding the actin filament are two thin strands of another protein, tropomyosin, that lie near the grooves between the actin strands. Each tropomyosin strand is itself a double helix as shown in Figure 31-15C.
The third protein of the thin filament is troponin, a complex of three globular proteins located at intervals along the filament. Troponin is a calcium-dependent switch that acts as the control point in the contraction process.
Sliding Filament Model of Muscle Contraction
In the 1950s the English physiologists A. F. Huxley and H. E. Huxley independently proposed the sliding filament model to explain striated muscle contraction. According to this model, the thick and thin filaments become linked together by molecular cross bridges, which act as levers to pull the filaments past each other. During contraction, cross bridges on the thick filaments swing rapidly back and forth, alternately attaching to and releasing from special receptor sites on the thin filaments, and drawing thin filaments past thick in a kind of ratchet action. As contraction continues, the Z lines are pulled closer together (Fig-ure 31-16). Thus the sarcomere shortens. Because all sarcomere units shorten together, the muscle contracts. Relaxation is a passive process. When cross bridges between the thick and thin filaments release, the sarcomeres are free to lengthen. This requires some force, which is usually supplied by antagonistic muscles or the force of gravity.
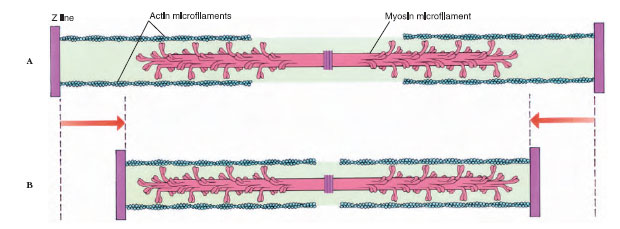 |
| Figure 31-16 Sliding myofilament model, showing how thick and thin myofilaments interact during contraction. A, Muscle relaxed. B, Muscle contracted. |
Control of Contraction
Muscle contracts in response to nerve stimulation. If the nerve supply to a muscle is severed, the muscle atrophies, or wastes away. Skeletal muscle fibers are innervated by motor neurons whose cell bodies are located in the spinal cord. Each cell body gives rise to a motor axon that leaves the spinal cord to travel by way of a peripheral nerve trunk to a muscle where it branches repeatedly into many terminal branches. Each terminal branch innervates a single muscle fiber. Depending on the type of muscle, a single motor axon may innervate as few as three or four muscle fibers (where very precise control is needed, such as the muscles that control eye movement) or as many as 2000 muscle fibers (where precise control is not required, such as large leg muscles). The motor neuron and all muscle fibers it innervates is called a motor unit. The motor unit is the functional unit of skeletal muscle. When a motor neuron fires, the action potential passes to all fibers of the motor unit and each is stimulated to contract simultaneously. Total force exerted by a muscle depends on the number of motor units activated. Precise control of movement is achieved by varying the number of motor units activated at any one time. A smooth and steady increase in muscle tension is produced by increasing the number of motor units brought into play; this is called motor unit recruitment.
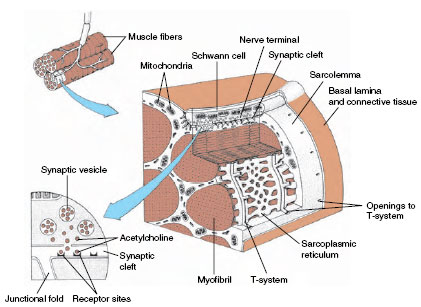 |
| Figure 31-17 Section of vertebrate skeletal muscle showing nerve-muscle synapse (myoneural junction), sarcoplasmic reticulum, and connecting transverse tubules (T-tubule system). Arrival of a nerve impulse at the synapse triggers the release of acetylcholine into synaptic cleft (inset at left). The binding of transmitter molecules to receptors generates membrane depolarization. This spreads across the sarcolemma, into the T-tubule system, and to the sarcoplasmic reticulum where the sudden release of calcium sets in motion the contractile machinery of the myofibril. |
The Myoneural Junction
The place where a motor axon terminates on a muscle fiber is called the myoneural junction (Figure 31-17). At the junction is a tiny gap, or synaptic cleft, that thinly separates a nerve fiber and muscle fiber. In the vicinity of the junction, the neuron stores a chemical, acetylcholine, in minute vesicles known as synaptic vesicles. Acetylcholine is released when a nerve impulse reaches a synapse. This substance is a chemical mediator that diffuses across the narrow junction and acts on the muscle fiber membrane to generate an electrical depolarization. The depolarization spreads rapidly through the muscle fiber, causing it to contract. Thus the synapse is a special chemical bridge that couples together the electrical activities of nerve and muscle fibers.
Built into vertebrate skeletal muscle is an elaborate conduction system that serves to carry the depolarization from the myoneural junction to the densely packed filaments within the fiber. Along the surface of the sarcolemma are numerous invaginations that project as a system of tubules into the muscle fiber. This is called the T-system (Figure 31-17). The T-system is continuous with the sarcoplasmic reticulum, a system of fluid-filled channels that runs parallel to the myofilaments. The system is ideally arranged for speeding the electrical depolarization from the myoneural junction to the myofilaments within the fiber.
Excitation-Contraction Coupling
How does electrical depolarization activate the contractile machinery? In resting, unstimulated muscle, shortening does not occur because thin tropomyosin strands surrounding the actin myofilaments lie in a position that prevents the myosin heads from attaching to actin. When muscle is stimulated and the electrical depolarization arrives at the sarcoplasmic reticulum surrounding the fibrils, calcium ions are released (Figure 31-17). Some calcium binds to the control protein troponin. Troponin immediately undergoes changes in shape that allow tropomyosin to move out of its blocking position, exposing active sites on the actin myofilaments. The myosin heads then bind to these sites, forming cross bridges between adjacent thick and thin myofilaments. This sets in motion an attach-pull-release cycle that occurs in a series of steps as shown in Figure 31-18. Release of bond energy from ATP activates the myosin head, which swings 45 degrees, at the same time releasing a molecule of ADP. This is the power stroke that pulls the actin filament a distance of about 10 nm, and it comes to an end when another ATP molecule binds to the myosin head, inactivating the site. Thus each cycle requires expenditure of energy in the form of ATP (Figure 31-18).
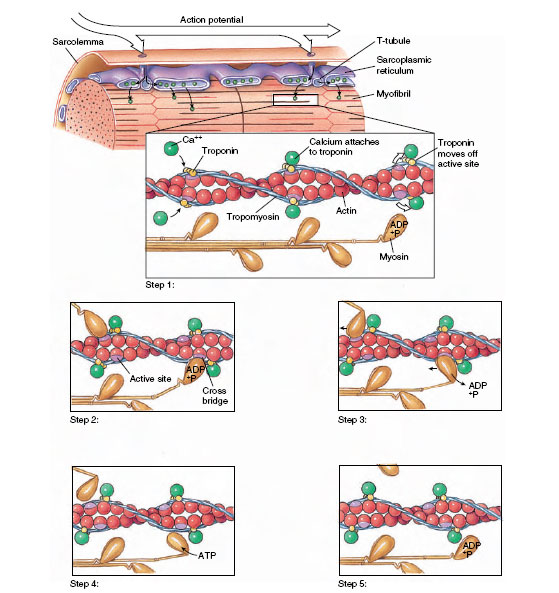 |
| Figure 37-18 Excitation-contraction coupling in vertebrate skeletal muscle. Step 1: An action potential spreads along the sarcolemma and is conducted inward to the sarcoplasmic reticulum by way of T tubules (T-tubule system). Calcium ions released from the sarcoplasmic reticulum diffuse rapidly into the myofibrils and bind to troponin molecules on the actin molecule. Troponin molecules are moved away from the active sites. Step 2: Myosin cross bridges bind to the exposed active sites. Step 3: Using the energy stored in ATP, the myosin head swings toward the center of the sarcomere. ADP and a phosphate group are released. Step 4: The myosin head binds another ATP molecule; this frees the myosin head from the active site on actin. Step 5: The myosin head splits ATP, retaining the energy released as well as the ADP and the phosphate group. The cycle can now be repeated as long as calcium is present to open active sites on the actin molecules. |
Shortening will continue as long as nerve impulses arrive at the myoneural junction and free calcium remains available around the myofilaments. The attach-pull-release cycle can repeat again and again, 50 to 100 times per second, pulling thick and thin filaments past each other. While the distance each sarcomere can shorten is very small, this distance is multiplied by the thousands of sarcomeres lying end to end in a muscle fiber. Consequently, a strongly contracting muscle may shorten by as much as one-third its resting length.
When stimulation stops, calcium is quickly pumped back into the sarcoplasmic reticulum. Troponin resumes its original configuration; tropomyosin moves back into its blocking position on actin, and the muscle relaxes.
Energy for Contraction
Muscle contraction requires large amounts of energy. ATP is the immediate source of energy, but the amount present will sustain contraction for only a second or two. Muscle cells immediately call on the second level of energy reserve, creatine phosphate. Creatine phosphate is a high-energy phosphate compound that stores bond energy during periods of rest. As ADP is produced during contraction, creatine phosphate releases its stored bond energy to convert ADP to ATP. This reaction can be summarized as:
Within a few seconds—perhaps as long as 30 seconds depending on the rapidity of muscle contraction—the reserves of creatine phosphate are depleted. The contracting muscle now must be fueled from its third and largest store of energy, glycogen. Glycogen is a polysaccharide chain of glucose molecules stored in both liver and muscle. Muscle has by far the larger store—some threefourths of all the glycogen in the body is stored in muscle. As a supply of energy for contraction, glycogen has three important advantages: it is relatively abundant, it can be mobilized quickly, and it can provide energy under anoxic conditions. As soon as the muscle’s store of creatine phosphate declines, enzymes break down glycogen, converting it into glucose-6- phosphate, the first stage of glycolysis that leads into mitochondrial respiration and the generation of ATP.
If muscular contraction is not too vigorous or too prolonged, the glucose released from glycogen can be completely oxidized to carbon dioxide and water by aerobic metabolism. During prolonged or heavy exercise, however, blood flow to the muscles, although greatly increased above the resting level, cannot supply oxygen to the mitochondria rapidly enough to complete oxidation of glucose. The contractile machinery then receives its energy largely by anaerobic glycolysis, a process that does not require oxygen. The ability to take advantage of this anaerobic pathway, although not nearly as efficient as the aerobic one, is of great importance; without it, all forms of heavy muscular exertion would be impossible.
During anaerobic glycolysis, glucose is degraded to lactic acid with release of energy. This is used to resynthesize creatine phosphate, which in turn passes the energy to ADP for the resynthesis of ATP. Lactic acid accumulates in the muscle and diffuses rapidly into the general circulation. If muscular exertion continues, the buildup of lactic acid causes enzyme inhibition and fatigue. Thus the anaerobic pathway is a self-limiting one, since continued heavy exertion leads to exhaustion. The muscles incur an oxygen debt because accumulated lactic acid must be oxidized by extra oxygen. After a period of exertion, oxygen consumption remains elevated until all of the lactic acid has been oxidized or resynthesized to glycogen.




