Bioengineering Strategies for Generating Plants with Modified Sterol Compositions
Plant metabolic engineering the isoprenoid–phytosterol pathway to understand
sterol biosynthesis and function has been underway for about 10 years. The
enzymes catalyzing the committed step in the isoprenoid and phytosterol pathways
are usually the most important control sites, and in plants they are the
HMGR and SMT enzymes, respectively (Bach, 1995; Holmberg
et al., 2003; Nes,
2000; Volkman, 2005). Phytosterol synthesis is likely controlled by allosteric interactions
involving end products of the pathway and nonsteroidal effectors,
changes in the amount of the SMT isoforms, compartmentation and interactions
between metabolically distinctive organs. The identification and biochemical
characterization of a number of Arabidopsis mutant lines (EMS mutants, T-DNA/Transposon insertion lines, transgenic plants) with an altered sterol
profile has enabled researchers in defining the distinct functional metabolic
units in the pathway. These mutants also point to the essential roles of sterols in
regulating plant development and morphogenesis (Benveniste, 2004). As the
genes encoding more enzymes of sterol synthesis are identified and manipulated,
it is becoming apparent that phytosterol homeostasis, carbon flux, and growth are
intimately tied to a variety of cellular functions and signaling pathways; therefore
careful metabolic manipulation of the pathways will be required to generate
value-added traits. From the biotechnological perspective the engineering of
SMT activity has yet to lead to a desired trait that can be commercialized. However,
considerable literature exists on the transgenic alteration of SMTs in plants,
which indicates agronomically important applications will be forthcoming.
Studies on the results of overexpression and underexpression of the SMT isoforms
in tobacco, tomato, potato, and Arabidopsis (Table 9.1) have recently confirmed
that SMT1 catalyzes the first step in the cycloartenol-sitosterol (Arnqvist
et al., 2003; Fonteneau
et al., 1977; Holmberg
et al., 2002; Schaeffer
et al., 2001;
Schaller
et al., 2001; Sitbon and Jonsson, 2001) and that SMT2 can regulate the levels
of 24-methyl sterol to 24-ethyl sterol in the plant (Arnqvist
et al., 2003). SMT1 from
plants is feedback inhibited by sitosterol but not by either ergosterol or cholesterol
(Nes, 2000). Alternatively, ATP serves as an activator of SMT1 (Nes, 2000).
Sitosterol inhibits the SMT1 in a competitive manner by decreasing its affinity for
substrates without affecting its V
max. Sitosterol can also inhibit SMT2 activity
but with significantly less effectiveness ca. K
i 100 µM versus 300 µM, respectively
(Parker and Nes, 1992). Pulse-chase experiments using radioactively labeled intermediates
(Bush and Grunwald, 1973; Heupel
et al., 1987; Nes, 1997; Nes, W. D. and
Nguyen, H. T., unpublished data) and microarray analysis (Ledford
et al., 2004)
have shown that light stimulates phytosterol synthesis and the expression of
SMT. Three strategies have been employed to genetically modify the phytosterol
composition in plants. These strategies are designed to either interrupt or
enhance carbon flux at the stage of SMT1 or SMT2 activity or to elaborate improved
enzymes aimed at reshaping enzyme specificities and mechanisms (Fig. 9.20).
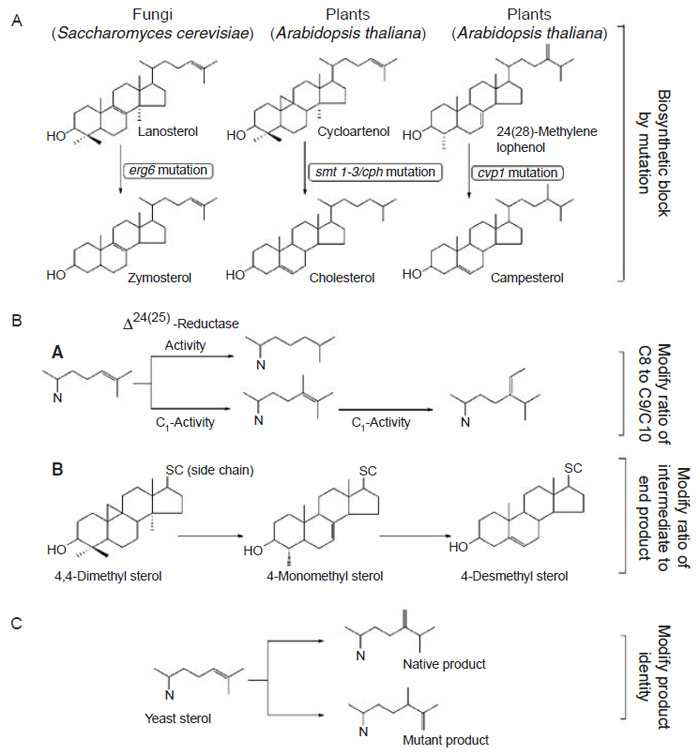 |
| FIGURE 9.20 Different strategies to engineer plants with modified sterol compositions.
(A) Engineer change in sterol composition through mutation; (B) engineer change in sterol
composition through antisense/cosuppression technology; (C) engineer change through
introduction of a foreign SMT that generates novel products. |
 |
| FIGURE 9.21 Comparison of
smt1 (Ac-mutagenized plant that
generates high levels of
cholesterol) and SMT1
(wild-type that generates high
levels of sitosterol) plants.
(A) Rosette of
2-week plants;
(B) rosette of 2-week-old smt1-
smt1–3 plant; (C) mature
(5-week) SMT1 and smt1–3
plant. Adapted from Diener et al.
(2000). |
The first approach to modify the phytosterol composition involves mutation of
a gene encoding SMT (Diener
et al., 2000). Single-enzyme mutation can result in an
inability to synthesize the enzyme in active form. Such a defect leads to a block in
the metabolic pathway at the point where the enzyme acts and the enzyme’s
substrate accumulates. In some cases the functional consequences of the mutation
have been investigated (Benveniste, 2004; Diener
et al., 2000). The
cvp1 Arabidopsis
plants defective in SMT2 activity accumulate campesterol, much the same way
erg6 yeast mutants defective in SMT1 activity accumulate zymosterol (Fig. 9.20,
Panel I) (Ledford
et al., 2004). The
smt1–3/
cph Arabidopsis plants defective in
SMT1 activity accumulate cholesterol rather than cycloartenol. The accumulation
of cholesterol (nonalkylated sterol) in significant amounts suggests enzymatic
reactions which normally do not recognize cycloartenol can process the intermediate
to a Δ
5-sterol in the transgenic plants. The induced mutations at the stage of
either SMT1 or SMT2 activity whereby a 24-desalkyl (C-8-sterol side-chain) sterol
accumulates or a 24-methyl (C-9-sterol side-chain) sterol accumulates agrees
with the order of intermediates positioned in the kinetically favored pathway of phytosterol biosynthesis. Mutant plants having a modified cholesterol to phytosterol
ratio are stunted consistent with the requirement for a fixed phytosterol
homeostasis (Fig. 9.21). In related work, inhibitors of sterol biosynthesis administered
to cultured plant cells (Nes
et al., 1991c) revealed a sequence of reactions in
the phytosterol pathway that posits SMT1 as a critical slow step. These transition
state analogs possess similar binding properties to either SMT1 or SMT2 (Nes
et al., 2003). Therefore, both SMT activities are impaired equally
in vivo as well as
in vitro. Studies in the design of inhibitors of SMT, activity have not yet progressed
to the stage where one compound can inhibit an individual SMT, although
mechanism-based inhibitors prepared in our laboratory are tailored with the
expectation to be SMT specific (Zhou
et al., 2004).
The second approach tomodify the phytosterol composition is to engineer plants
with an SMT construct in the sense or antisense direction either of which can lead to
cosuppression of native SMT synthesis (Fig. 9.21, Panel B). Phytosterol regulation
can favor different branch point enzymes to generate a sterol mixture containing
side-chains of varying degrees of C-24 alkylation. If carbon flux in phytosterol
synthesis passes along the same set of tracks separated by SMT1 and SMT2, equilibrium
considerations will dictate that either slowing or increasing the traffic in one
direction by modifyingSMT expression will lead to a change in the ratio of C-8- to C-
9- to C-10-sterol side-chains (Table 9.1). The simplest approach to disturb the steady
state concentration of SMT is to engineer either a sense or antisense construct of the
native SMT1 to a plant. Transgenic tobacco plants with modified expression of
the native SMT1 gene were prepared by introducing sense and antisense expression cassettes of cDNANtSMT1–1 to tobacco plants (Arnqvist
et al., 2003;Holmberg
et al., 2002; Schaeffer
et al., 2001; Sitbon and Jonsson, 2001). The resulting plants possessed
variations in the cycloartenol proportions and a concomitant effect on the proportion
of 24-ethyl sterols. In these plants, the total amount of sterol remained relatively
unchanged, consistent with our hypothesis that plants will tend to maintain a sterol
homeostasis to the extent possible (Nes, 1990). Arabidopsis plants showing cosuppression
of SMT2–1 were characterized by high campesterol levels and depletion of
sitosterol (Sitbon and Jonsson, 2001). Pleiotropic effects on development such as
reduced growth appear to result from changes in expression of SMT1 and SMT2
(Benveniste, 2004; Lindsey
et al., 2003; Schaller, 2004). In a related study, an Arabidopsis
frill1 (fri1)mutantwas generated that had amutation inSMT2 and an altered
C
1/C
2-methyl sterol composition. Petalmorphogenesiswas found to be impaired by
the change in phytosterol homeostasis (Hase
et al., 2005). Overexpression of soybean
SMT1 in transgenic potato plants results in a marked reduction of cholesterol and
glycoalkaloids (Sitbon and Jonsson, 2001), consistent with the role ofSMT1 to control
the level of C-8- to C-9/C-10-sterols.
The reports published thus far on engineering modified sterol pathways in
plants revolve around engineering a plant SMT back into plants. An alternative
strategy adopted in our laboratory (Nes and Nguyen unpublished data) is to
engineer a fungal SMT into plants. We were concerned initially whether plants
would either express or otherwise tolerate a fungal SMT. This could be due to either
genetic mechanism or due to a failure of the enzyme to catalyze plant substrates
which normally are unacceptable to the plant SMT. According to structure–activity
tests with the yeast SMT, neither cycloartenol nor 24(28)-methylene lophenol will
bind productively (Zhou and Nes, 2003), although zymosterol, the optimal substrate
for the yeast SMT1, is a good substrate for SMT2. These findings led to the
hypothesis that engineering a yeast SMT to plants will promote the underexpression
of the native SMT1 by cosuppression and interrupt carbon flux to SMT2 by
providing a foreign SMT that can compete for substrates targeted for SMT catalysis.
If substrates normally converted by the plant SMT isoforms were acceptable to the
yeast SMT1, then it might be possible to engineer plants with a yeast mutant SMT1
(or some related SMT) capable to generate novel products (Fig. 9.21, Panel C).
To study our hypothesis further, two different ERG6 SMT constructs were
developed. The first ERG6 SMT construct was developed as a consequence of PCR
modification which gave rise to a mutation that introduced a frameshift in the
gene toward the N-terminus (Nes, unpublished data). This
Saccharomyces cerevisiae SMT1 containing a frameshift mutation is referred to as ScSMT1-FSM. A
second ERG6 SMT construct was developed from site-directed mutagenesis of
an amino acid at position-81 which corresponds to the sterol binding site (Nes
et al., 1999). This
S. cerevisiae SMT1 containing a tyrosine to phenylalanine mutation
at position-81, referred to as ScSMT1-Y81F, was chosen for the engineering
studies because it has altered substrate specificity and product sets that make it
plant-like. Tomato plants harboring the ScSMT1-FSM transgene contained a high
level of cycloartenol and a corresponding decreased level of 24-ethyl sterols
(Table 9.2). Tobacco plants harboring the Y81F yeast mutant contained decreased
levels of cholesterol and increased levels of 24-ethyl sterols. It is expected that overexpressing a foreign SMT1 in plants will increase the overall SMT1 activity
(resulting from the combination of native protein and transgene protein) thereby
increasing flux after the formation of 24(28)-methylene cycloartenol, which
appears to be the case for either plant or fungal SMTs engineered into plants.
The ScSMT1-FSM was not expressed in tomato whereas the Y81F yeast mutant
was constitutively expressed, as determined by several techniques including
activity assay, Northern blot analysis, and immunochemistry using the yeast
SMT antibody (unpublished data). The overexpressed yeast SMT1-Y81F did not
appear to compete for endogenous substrates of tobacco since the plant did not
make any acceptors suitable for the fungal SMT catalysis.
 |
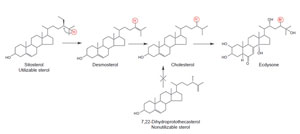 |
| FIGURE 9.22 Conversion of sitosterol to
cholesterol and ecdysone by phytophagous
insects.
The hydrogen circled in red migrates from
C-25 in sitosterol to C-24 in cholesterol during
the
C-24-dealkylation reaction. The methyl group
at C-24 in 7,22-dihydroprotothecasterol remains
intact for mechanistic reasons. |
Because it is generally undesirable to alter the balance of 4,4-dimethyl sterol
intermediate to 4-desmethyl sterol end products, except according to the normal
developmental program, single-enzyme manipulations designed to either
increase the amount of intermediates or change the ratio of C-24-alkylated sterols
in the sterol mixture (Fig. 9.21, Panel B), are limited to upregulating HMGR
activity and downregulating SMT activity (Bach, 1995; Holmberg
et al., 2003;
Guo
et al., 1995). Generally, changes in the intermediate to end product ratio result
in cycloartenol accumulating as sterol ester in lipid droplets whereas the modified
ratio of 24-methyl to 24-ethyl Δ
5-sterols results in little change in the total cellular
sterol thereby maintaining sterol balance in the membrane.
The third approach being developed in our laboratory is to engineer a combination
of transgenes in the antisense and sense directions to crop plants. We expect to eliminate the expression of the native SMT1 thereby permitting a foreign
SMT to be expressed that possesses an unusual catalytic
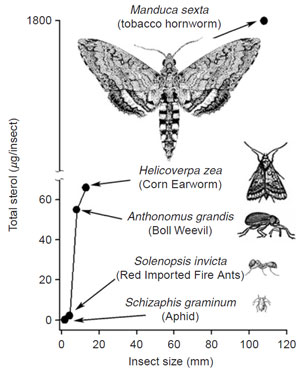 |
| FIGURE 9.23 Correlation of sterol content with
insect size. Adapted from Behmer and Nes,
2003. |
competence (Fig. 9.21,
Panel C). This strategy is based on the observation that insects do not synthesize
their own sterol and require cholesterol to synthesize ecdysteroid involved in the
molting process (Behmer and Nes, 2003). Phytophagous insects convert sitosterol,
obtained from the host plant, to cholesterol (Fig. 9.22) (Behmer and Nes, 2003; Nes
et al., 1997). Insects, like plants, have different sterol requirements depending on size and with respect to their phylogeny (Fig. 9.23). In the C-24-dealkylation
pathway, the hydrogen atom at C-25 migrates to C-24 during the elimination
of the 24-ethyl group. Thus, phytosterols with a Δ
25(27)-bond will not undergo
side-chain metabolism by the insect; therefore these sterols will be nonutilizable
nutrients for growth (Svoboda
et al., 1995 Nes
et al., 1997). In order to develop
insect-resistant plants, a strategy we considered is to redesign the yeast SMT1 to
affect its catalytic competence in such a way to bind cycloartenol, to remove the
affinity for effectors (sitosterol) that might interfere with SMT activity, and to
modify the active site to promote channeling to generate Δ
25(27)-olefins. It is clear
that we can tailor SMTs to produce new substrate affinities and products. The
opportunities to generate transgenic plants that appear similar to wild types with
modified sterol compositions are limitless and soon the commercial benefits of
this evolving bioengineering technology directed at phytosterols will be realized.