Phytosterolomics
The study of metabolic networks and feedback response to developmental and
environmental conditions involves the profiling of natural products. In this context,
phytosterolomic research can be defined as the complete identification and
quantification of all sterols present in a specific biological sample. Phytosterol
fingerprinting is another aspect of phytosterolomics where the unusual sterol
composition of a pathogen can serve as a signature lipid for disease such as for
immunocompromised patients harboring pneumocystis (Kaneshiro
et al., 2002;
Zhou
et al., 2002). Phytosterol profiling involves techniques such as thin layer
chromatography (TLC) and high-performance liquid chromatography (LC) mass
spectroscopy (LC/MS) and gas chromatography mass spectroscopy (GC/MS)
where individual components are separated and tentatively identified and quantified
by one set of techniques with final characterization of structure determined
by
1H or
13C NMR spectroscopy (Kalinowska
et al., 1990; Nes
et al., 1998a, 1999,
2003). HPLC equipped with a diode array detector to monitor UV is useful in
the identification of the type and amount of conjugation in the sterol molecule
(Nes
et al., 1985; Norton and Nes, 1991; Xu
et al., 1988).
Separation factors in GLC
and HPLC based on the retention of cholesterol relative to the retention time
of sterol standards have been reported to enable the prediction of the identity of
an unknown sterol (Xu
et al., 1988). High-throughput screening of phytosterol mixtures by conventional chromatography and spectroscopy has not yet progressed
to the point where a single method can establish the identities of a
complicated set of structurally similar phytosterols. Although the sterol patterns
of many plant extracts deduced by GLC are rather simple, usually consisting
of three or four major sterols, the sterol composition of specific plant parts or
the type of sterol in primitive versus advanced plants can be strikingly different
(Nes, 1990).
The sterol side-chain can be modified by the addition of one or two supernumerary
carbon atoms at C-24 with either α- or β-chirality. Establishing the
stereochemistry of the 24-alkyl group of individual sterols is a formidable task
and can be achieved by identifying specific side-chain carbons by high-field NMR
(Fig. 9.7, Panels A–C). The assignments of the signals from the
1H or
13C
NMR spectra of
1H- or
13C-labeled sterols are diagnostic for the origin of the
methyl group at C-24 and the biosynthetic stereospecificity of phytosterol sidechains
(Guo
et al., 1996, 1995; Popják
et al., 1977). The relative proportion of α- to
β-isomer of a 24α/β-methyl cholesterol mixture can also be quantified by reversedphase
HPLC (Guo
et al., 1995) (Fig. 9.7, Panel D) (Parker and Nes, 1992). The ratio
of these diastereoisomers can change as plants evolve from less to more advanced
(Nes
et al., 1977).
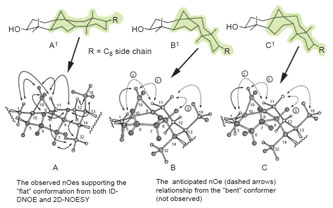 |
| FIGURE 9.8 NOE networks that indicate whether
cycloartenol can orient into a flat or bent
shape.
Adapted from Nes et al. (1998b). |
A more exacting analysis of sterol structure and stereochemistry involves the
use of several spectroscopic techniques, including high-field
1H and/or
13C-NMR,
X-ray crystallography, as well as molecular modeling (Nes
et al., 1998a). An
example where the use of several techniques has played a major role in our
understanding of conformational analysis of plant sterols is the characterization
of cycloartenol (with a 9β,19-cyclopropane ring) and lanosterol (with a Δ
8(9)-
bond). There is a hypothesis that the structural isomers are shaped differently,
bent versus flat (Bloch, 1983; Goodwin, 1981; Rahier
et al., 1984). The putative bent
9β,19-cyclosterol was considered to be an unique structural trait of intermediate
plant sterols, and bent compounds were acceptable only to either specific plant
enzymes or membrane systems. The rationale for 9β,19-cyclosterols to be bent is
based on the
syn–cis configuration at theA/B and B/C ring junctions, resulting in an
unfavorable interaction between the 9,10-bridgehead
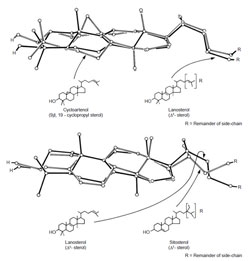 |
| FIGURE 9.9 Partial X-ray
crystallographic structures of
cycloartenol, lanosterol, and sitosterol.
Adapted from Nes et al. (1991b). |
and the 8β-hydrogen atomat
C-8. Furthermore, on the basis of manipulation of ball and stick models, it was
proposed that ring B in 9β,19-cyclosterols becomes a boat and theA/B/Crings orient
in the chair-boat-chair conformation. However, we discovered for the first time
excellent agreement between a set of crystallographically observed cycloartenoltype
structures and their solution conformations deduced from 2D-NMR spectroscopic
analysis, analysis of the NOE networks (Fig. 9.8) and MM/MD calculations
to be pseudoplanar (A/B/C rings are chair/half-chair/twist-chair conformer), and
the sterol side-chain to orient to the ‘‘right’’ (Nes
et al., 1984, 1991a). These results
with sitosterol, lanosterol, and related compounds reveal that the compounds
possess similar three-dimensional shapes (Nes
et al., 1991b) and differ in the tilt of
the C-3 group and C-17(20) side-chain (Fig. 9.9), structural features that can be
responsible for the different activities of sterols (Nes
et al., 1991b, 1993).
Using the modern methods of sterol analysis, it has been possible to show that
phytosterols appear in all cells at all stages of development, but the type and amount of sterol is greatly influenced by ontogeny and speciation, and consequently
seems to be a carefully regulated process (Nes, 1990). In spite of findings
that show variability of the sterol side C-24 alkyl structure occurs widely, suggesting
some sort of association between sterol structure and plant biology, relatively little is known about individual sterols or sterol sets and their role in plant
physiology.
As many as 60 sterols have been reported in a vascular plant,
Zea mays (Guo
et al., 1995), which is about one-third to one-half the number of
phytosterols found in living systems; 39 sterols have also been identified in an
ascomycetous fungus,
Gibberella fujikuroi (Nes
et al., 1989a).
The ratio of 4,4-dimethyl and 4-desmethyl sterol intermediate to 4-desmethyl
sterol end product, easily determined by TLC, can be as much as 1:3 in pollen,
1:3 in the seed, and 1:9 in vegetative parts (Heupel
et al., 1986; Marshall
et al., 2001;
Nes and Schmidt, 1988; Nes
et al., 1991c). These differences in intermediate to end
product ratios suggest that the phytosterol pathway is undergoing significant
changes during development. Consistent with this observation, as plants mature
from seed to flower the amounts of total sterol in individual parts and various cell
types differ dramatically. For example, in sunflower the sterol levels are reported
to be (Nes, 1990, 1991b,c) in: a seed, 83 µg; the 4-day dark grown sprout, 15 µg; the
primary leaf, 15 µg; mature leaf, 100 mg; immature green flower bud, 35 µg; disk
flower, 11 µg; cultured cells, 3,000 fg/cell; and the ray mesophyll cells released by
macerase digestion, 500 fg/cell. Pollen grains were found to be the richest source
of sterol, 585,000 fg/grain.
Sterols accumulate in vegetative parts mostly as 24-ethylsterols (e.g., sitosterol
and stigmasterol [22-dehydrositosterol]) whereas pollen can accumulate significant
amounts of 24-methylene sterols and 24-desalkylsterols (4,4-dinorcycloartenol;
24-dehydropollinastanol) (Guo
et al., 1995; Nes and Schmidt, 1988). However, the sterols of some pollen and seeds can be mostly sitosterol and related phytosterols.
Stem trichomes separated from sunflower had no detectable sterol within a
GLC limit of detection set at <0.1% total sterol. Alternatively, the leaf wax contains
cholesterol, as reported for sorghum leaves (Nes, 1990). Cycloartenol, usually an
intermediate that accumulates to trace levels in the sterol mixture, can accumulate
to as much as 25% in aging tomato leaves (unpublished data) and similarly can be
a major sterol in soybean seeds (Marshall
et al., 2001). The inability to detect
cycloartenol in some instances may be due to the manner in which sterol was
prepared for analysis. For instance, in many early studies sterol purification
involved digitonin precipitation that fails to precipitate 4,4-dimethyl sterols
along with the major phytosterols; therefore only the Δ
5-sterol content in the
tissues was determined.
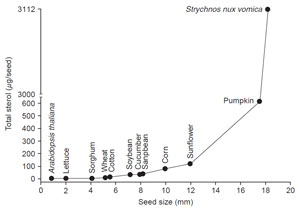 |
| FIGURE 9.10 Correlation of the sterol content
with the increase in size of seeds.
From
unpublished data. |
In contrast to other lipids, a correlation exists between the amount of sterol
and seed size. Thus, as the seed increases in size the amount of sterol increases from as little as a few micrograms per seed in Arabidopsis to approximately
3,000 µg/seed in
Strychnos nux vomica (Fig. 9.10). In similar fashion, the amount
of sterol in vegetative plant parts (e.g., leaves) increases as the blade size increases
during growth and as the plant height approaches maturity (Fig. 9.11). It would
appear that the free sterol content of a system is roughly related to the cell number;
therefore the amount of total sterol of a system can be an approximate measure of
the total number of cells. Thus, Arabidopsis seeds will contain a smaller number
of cells than Strychnos seeds.
The amount of total phytosterol in seedlings has been correlated to the level of
SMT activity during plant maturation (Fig. 9.12). Transcript expression levels
of SMT from Arabidopsis and soybean also vary during plant maturation
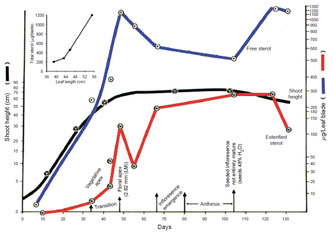 |
| FIGURE 9.11 Correlation of the sterol content with the
increase in shoot height of sorghum.
Inset is a
correlation of the increase in sterol content with
maturation of the leaf blade, measured
as changes in
leaf length. Adapted from Heupel et al. (1986). |
(Carland
et al., 2002; Diener
et al., 2000; Shi
et al., 1996). For example, young
roots, leaves, and stems had higher levels of steady state transcript levels as
compared with mature leaves, suggesting a high rate of sterol biosynthesis in
growing vegetative tissues. Growing apical meristems appear to be a major site
for sterol biosynthesis (Devarenne
et al., 2002). In plants, phytosterol synthesis
is measurably a slow event, ca. 1.0 pmol/h/100 shoots, consistent with their primary
function as architectural components of membranes, and cycloartenol is
turned over rapidly at 24 pmol/h/mg protein consistent with its role as an intermediate
(Guo
et al., 1995). Thus, to meet the continued needs of campesterol and
sitosterol formation, carbon from the isoprenoid pathway must be continually
made available to cycloartenol synthesis.
The phytosterol composition can change in regard to the structure of the sterol
side chains with plant development. A ‘‘switching’’ mechanism in the biosynthesis
of phytosterols has been shown in the cucurbits to produce different 24-alkyl sterol stereoisomers with
development (Kalinowska
et al., 1990). The
seeds of squash and pumpkin synthesize 24β-ethyl sterols whereas the seedlings
of
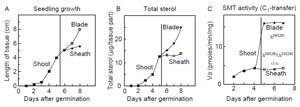 |
| FIGURE 9.12 Correlation of the rate of
phytosterol synthesis with plant growth and
SMT
activity. Data adapted from Guo et al. (1995). |
these plants synthesize 24α-ethyl sterols (Nes, 1987a). Similarly, the ratio of
24α-ethyl sterol to 24β-ethyl sterol can switch in Kalanchoe; roots (total sterol about
285 µg/fwt) contain exclusively the α-isomer and leaves (total sterol 232 µg/fwt)
contain the β-isomer. Flowers (total sterol 160 µg/fwt) have an equal mixture of
24α/β-ethyl sterols. These profiling results strongly suggest that the level and
perhaps type of SMT expressed in individual cells and cell types during ontogeny
is regulated differentially and is an important determinant of phytosterol
diversity.
The principal approach adopted seeks to derive developmental and evolutionary
understanding of sterol synthesis by combining the sterol and genetic
composition of plants with sterol functions and the morphological evidence of
plants at different time points. These studies indicate that sterol biosynthesis can
proceed in plants by a cycloartenol pathway and in animals and fungi by a
lanosterol pathway (Goodwin, 1981; Nes
et al., 1990). In addition, the size and
direction of the 24-alkyl group of the sterol side-chain can be an indicator of an
early or late stage of development and whether the plants are less or more
advanced. Primitive organisms synthesize phytosterols with a 24β-methyl group
and vascular plants synthesize sterols with a 24α-ethyl group (Kalinowska
et al., 1990). Since C-methylation is an energy expensive process, it seems unlikely that
the cell would commit energy to produce the side-chains of ergosterol or sitosterol
unless the methylation event was functionally significant. Although few pure
enzymes of sterol catalysis have been examined in any detail, it does seem safe
to suggest that a relatively small number of SMT enzymes determine the basic
structural character of the phytosterol side chains produced in a given species.
The type and amount of phytosterol can change during the life history of
plants and fungi yet there is a sterol homeostasis maintained at the cell level
throughout development. The question arises as to why certain sterols accumulate
and what biology maintains their balance in the cell. In other words, is one sterol
as good as another? Can a single sterol play multiple roles? Is there something
special about a particular sterol cocktail? One way to get at this problem would be
to determine what sterols actually do, to find a way to quantify the value of
individual features, and then to determine the extent to which deviations in sterol
homeostasis affect function. The occurrence of intermediates is also problematic
since different pathways can lead to the same end product. In the first case, the
biosynthetic sequence determines the structure of the end product. In the second
case, a choice presumably is made in a way that has no impact on the structure
of the end product. Nes discussed the possible multiple roles of sterols in plants
and fungi (Nes, 1980) and the difference between functional and phylogenetic
control of biosynthesis (Parker and Nes, 1992). Evidence in support of phylogenetic
control is the cycloartenol-lanosterol bifurcation whereby either precursor
can give rise to ergosterol. In contrast, functional control is related to the structure
and function of enzymes that act on sterols that, once impaired, interrupt flux to
end products resulting in aberrant morphology or cell death. In support of the
latter hypothesis is recent work from animal and plant studies that show mutations
in the terminal segments of the cholesterol and sitosterol pathway lead to
malformations (Clouse, 2002; Herman, 2003).
The hypothesis that sterols play multiple roles in plants and fungi has its
origins with the work of Clark and Bloch (1959) who studied the insect nutritional
requirements of sterol. Insects cannot make their own sterol due to a block in the
pathway before squalene oxide cyclization, therefore they are dependent on an
exogenous or dietary source of sterol to satisfy their structural and physiological
needs for them (Clark and Bloch, 1959). Clark and Bloch discovered a sterol
function other than the bulk membrane role for sterols on the basis of structural
and quantitative requirements. Thus, feeding different amounts of a mixture of cholesterol and cholestanol to the insect resulted in the finding that cholestanol
can spare (partly replace) cholesterol. The function of cholestanol was considered
to be that of a regulatory molecule, not necessarily as a precursor to a hormone, for
example, ecdysteroid. Fungi have been found to utilize sterols in multiple roles as
well and various actions similar to sparing cholesterol in insects have been
described with the yeast ergosterol (Nes, 1987b). The requirement for sparing
amounts of a 24-ethyl sterol was demonstrated using cultured celery cells
(Haughan
et al., 1987). Support for the involvement of the different phytosterols
in different physiological roles has been obtained with the fungus
G. fujikuroi where there is delayed expression of certain sterolic enzymes that generate different
sterol compositions (Nes and Heupel, 1986) and mutation studies in plant
sterol synthesis that show changes in sterol composition affects morphology
(Lindsey
et al., 2003; Schaller, 2004).









