Enzymology and Evolution of the SMT
SMTs are a family of AdoMet-dependent C-methyltransferases that use two
substrates, AdoMet as a methyl source and sterols with a Δ
24-bond as the acceptor
molecules, for the transmethylating reaction yielding AdoHcy and phytosterols
with single or double methylation at C-24. AdoMet-dependent methylations
are important in generating phytosterols as primary metabolites, and other
AdoMet-dependent methylations contribute to generating many secondary products,
including phenylpropanoids, flavonoids, and alkaloids (Nes
et al., 1986;
Ounaroon
et al., 2002; Schubert
et al., 2003; Zubieta
et al., 2002). The primary
structures and enzyme kinetics as well as the requirements for the substrate
may be quite different among the methyltransferases. However, a related evolution
may be inferred to the generation of these seemingly different plant enzymes
in which the active center evolved a common core of structurally similar amino
acid residues for interaction with AdoMet.
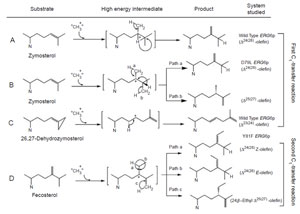
SMT-catalyzed reactions are remarkable in that they convert lipophilic
compounds to methylated olefins in a single step. The details of these processes
have intrigued scientists for half a century. A comparison of
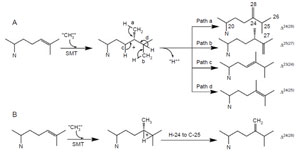 |
| FIGURE 9.13 Possible C-methylation
mechanisms for producing C-28 olefins. (A)
Stepwise or
carbocationic pathway; (B) nonstop
or concerted pathway. |
biomimetic studies of
uncatalyzed versus SMT-catalyzed reactions reveal that the elementary chemical
steps required for C-methylation of an olefin can take place to produce multiple
products in a nonezymatic reaction following predictable chemical principles (Julia
and Marazano, 1985; Venkatramesh
et al., 1996). Mechanistically, the key to the
C-methylation reaction is the positively charged sulfoniumcenter in AdoMet which
renders the methyl group electrophilic and susceptible to the relatively nucleophilic
Δ
24-double bond. The ensuing reaction proceeds by a reorganization of at least
three bonds: (1) cleavage of the C–S bond in the AdoMet donor, (2) formation of the
C-24(28)-bond between the donor and the acceptor, and (3) loss of a proton from
either the donor or acceptor. SMT catalysis proceeds stereo- and regiospecifically to
generate distinct product sets by variations of a similar ionic mechanism (Fig. 9.13).
Together these enzymes are capable of converting sterol precursor into more than
100 distinct phytosterols in plants (Nes and McKean, 1977).
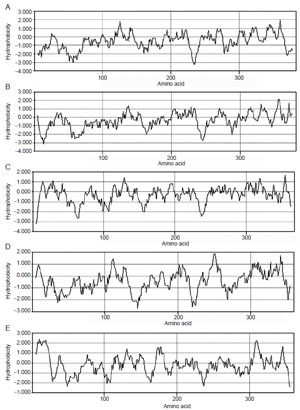 |
| FIGURE 9.14 Hydropathy plots corresponding
to SMTs for (A) S. cerevisiae; (B) Gibberella
fujikuroi; (C) Glycine max; (D) Trypanosoma
brucei; (E) Arabidopsis thaliana. |
SMTs are membrane-bound enzymes which share a high degree of similarity
in primary structure as revealed by a comparison of their amino sequences
reported in the GenBank. Hydropathy analyses of SMTs from different organisms
indicate that they are similar and moderately hydrophobic with no membrane
spanning domains (Fig. 9.14). Several cloned SMT enzymes from plants, fungi,
and protozoa have been overexpressed in
Escherichia coli (Nes
et al., 1998b, 2003;
Zhou
et al., 2006). The open reading frame of these catalysts will code for predicted
proteins of 336–383 amino acids with a molecular mass that ranges from 38.5 to
43.3 kDa. In all cases studied, the purified protein possesses a tetrameric subunit
organization that ranges from 160 to 172 kDa. Equilibrium dialysis and Scatchard
plotting of K
d measurements indicate that the SMT has a single binding site for
sterol and AdoMet. Catalytic constants for the native substrates for these enzymes
are generally K
m ca. 30 µM and k
cat ca. 0.01 s
—1. The secondary structure of several
SMT1 enzymes, as determined by circular dichroism, was found to be similar
(Nes
et al., 2004; Zhou and Nes, 2003). In the case of the yeast SMT, the following
population of structures was recorded: 43% α-helix, 29% β-sheet, 7% turn, and
21% random coil (Zhou and Nes, 2003). These findings suggest the conformational
features of the native SMTs will be similar. Although X-ray crystallographic
analysis of heavy atom-labeled crystalline SMT bound to a suicide substrate
could provide much helpful information about structure–function relationships,
crystallization of the SMT has proven to be a formidable task and no threedimensional
structure of the enzyme is available to date. Despite this fact, sufficient
activity assays of substrate and transition state analogs with different
SMTs are available to speculate a
steric–electric model of SMT catalysis (Fig. 9.15)
(Parker and Nes, 1992).
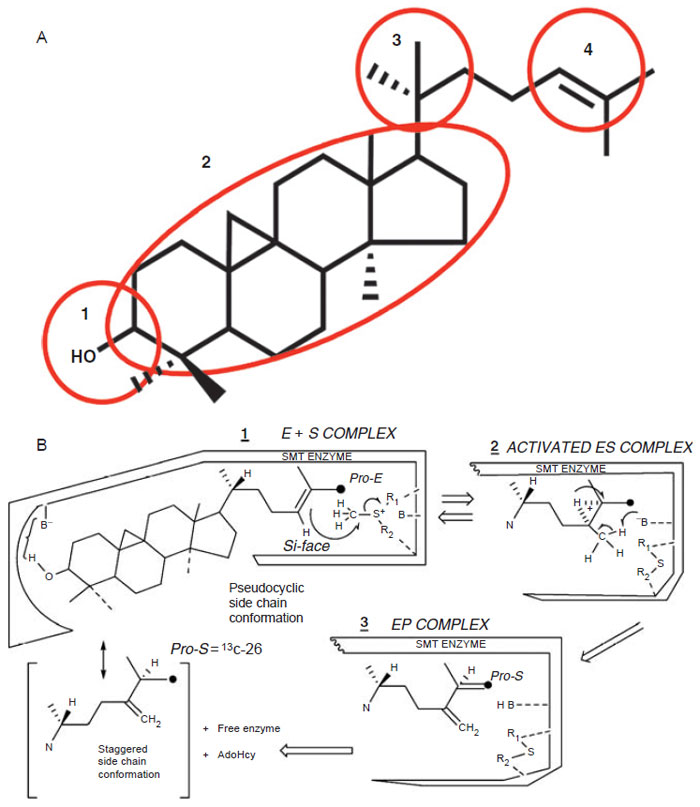 |
| FIGURE 9.15 Postulated domains of sterol molecule (Top, A) recognized by the SMT and the steric–electric plug model of SMT catalysis (Bottom, B). |
The model predicts that four domains of the sterol molecule are critical to
productive binding and catalysis. Domain 1 recognizes an equatorial C
3–OH
group that is nucleophilic to bind to a polar amino acid; Domain 2 recognizes a double bond in the nucleus, preferable at Δ
8(9) (or a 9β,19 cyclopropane group),
to lock the nucleus in a pseudoplanar conformation thereby generating a flat
shape and interacts with the angular methyl groups at C-10 and C-13 to secure
the sterol in the hydrophobic cleft; Domain 3 recognizes a 20R-configuration
to direct the side-chain into a ‘‘right-handed’’ conformation thereby positioning C-22–C-26 to a catalytically acceptable conformation; and Domain 4 recognizes
a side-chain that contains the terminal C-25-C-26–C-27-isopropyl group and
Δ
24-bond to anchor the side-chain near AdoMet. The model also predicts a
conformational change in the enzyme during catalysis to allow for the different
kinetics of the reaction (Parker and Nes, 1992).
For the first C
1-activity catalyzed by the yeast SMT, the methyl transfer reaction
proceeds by a simple S
N2 reaction involving a dative bond formed between
C-24 and C-28 and a bridged carbenium ion formed opposite to the side of the
original double bond facing the AdoMet. A 1,2-hydride shift of H-24 to C-25
proceeds because both C-24 and C-25 have become tertiary sites, equally stabilized
by hyperconjugation. The hydride shift of H-24 to C-25 and methyl transfer (to C-24 from AdoMet) are concerted; only in this way will there be no formation
of a discrete C-24 or C-25 cation and channeling will be restricted to formation of
a single product (Nes
et al., 1998b; Nes
et al., 2003).
The plant SMT1, in contrast to the yeast SMT1, can catalyze both the first
and second C
1-transfer reaction. The first C
1-activity acts mechanistically similar
to the yeast SMT1 to produce a Δ
24(28)-product. However, the second C
1-activity
can generate a mixture of 24-ethyl sterols by an ionic mechanism involving a C-24
isofucosterol cation and a reversible 1,2-hydride shift of H-24 to C-25 that
leads to deprotonation and formation of different olefins corresponding to
the side-chains of fucosterol (Δ
24(28)E), isofucosterol (Δ
24(28)Z), and clerosterol
(Δ
25(27) 24β-ethyl) (Nes
et al., 2003). The chemical mechanisms associated with
the first and second C
1-activities differ in the order the two bonds are cleaved
in the Δ
24(25)- and Δ
24(28)-substrates. In the carbocation mechanism, the bond to
the leaving group is broken first whereas in the concerted mechanism, both bonds
are cleaved simultaneously without intervention of an intermediate. Thus, the
second C
1-activity can operate a stepwise mechanism whereas the first C
1-activity
can operate a nonstop mechanism. In similar fashion, the first C
1-transfer can
proceed by a stepwise mechanism to produce multiple products, as recently found
for the cloned
Trypanosoma brucei SMT that converts the Δ
24(25)- sterol to a mixture
of Δ
24(28)-, Δ
25(27)-, and Δ
24(25)-sterols (Zhou
et al., 2006). The operation of path a in
the yeast SMT compared to the operation of paths a and b versus path d in the
T. brucei SMT (Fig. 9.13) suggests that the yeast SMT can operate a 1-base mechanism
whereas the
T. brucei SMT can operate a 2-base mechanism for the coupled
methylation–deprotonation reaction.
Studies on a set of cloned wild-type and mutant yeast SMT (Nes
et al., 1999;
Nes
et al., 2002; Zhou and Nes, 2003) indicate that the conserved acidic amino
acids at D125 and D152 form a wall of the AdoMet binding site, perhaps hydrogen
bonding to the methionine and ribose moieties of the substrate; D276 and E195 are
positioned directly or by way of a water bridge to the proximal (C
3-hydroxyl
group of the sterol) and distal (Δ
24-bond of the sterol) nucleophilic segments of
the acceptor, respectively. E195 may also interact with the positive charge on the
sulfur residue of AdoMet in which case it may serve as a counterion to AdoMet.
Alternatively, through cation-π interactions, a neighboring aromatic amino
acid may serve as the counterion to AdoMet. H90, positioned above (
Si-face of
the 24,25-double bond) the substrate double bond in the same plane as AdoMet,
may serve as the base involved with C-28 deprotonation that leads CH
3 to CH
2 production. The residue at Y81 may lie on the
Re-face of the substrate double
bond and act during the methylation-deprotonation reaction to stabilize the
high-energy intermediate(s) formed during the reaction progress. Negatively
charged ions included in the SMTs are considered to be arranged in the active
site so as to stabilize the intermediary carbocations formed during catalysis
through cation-π interactions (Nes
et al., 2004), thereby restricting channeling
and leading to an acceleration of the C-methylation reaction. Homology modeling
and the enzymatic studies with SMT predict a spatial arrangement of the secondary
structural elements in relation to sterol and AdoMet substrates as shown in
Fig. 9.16.
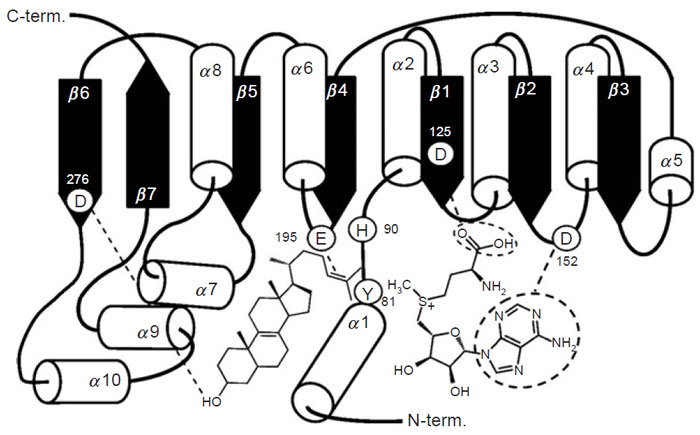 |
| FIGURE 9.16 Schematic representation of the methyltransferase fold of the SMT; spatial
arrangement of the secondary structure elements in relation to sterol and AdoMet substrates.
Adapted from Nes et al., 2004. |
Recent developments in the cloning and purification of SMTs and studies
performed in our laboratory (Mangla and Nes, 2000; Nes, 2000; Nes
et al., 2003;
Zhou
et al., 2006) and that of Benventiste (2004) have led the enzyme commission
(EC) to reclassify the SMT family according to substrate preference into three
classes: the fungal SMT1 prefers zymosterol [SMT1
ZY], EC 2.1.1.41; the plant
SMT1 prefers cycloartenol [SMT1
CA], EC 2.1.1.142; and the plant SMT2 prefers
24(28)-methylene lophenol [SMT2
ML], EC 2.1.1.143. SMT1 and SMT2 catalyze the
first and second methylation activities, respectively. If two enzymes belong to the
same class in this classification, they are considered to have similar chemical
functions. To date, the EC does not recognize the ability of SMTs to catalyze
different product distributions as a measure of function. Moreover, there must
be additional SMTs with substrate preferences not heretofore recognized by the
EC. For example, fungi exist that accumulate lanosterol rather than zymosterol
when treated with an inhibitor of SMT (Nes
et al., 2002), suggesting these catalysts,
referred to as SMT1
LA, prefer lanosterol to zymosterol.
The
steric–electric plug recognizes structural complementarity between the
SMT and the substrate molecules. However, the substrate affinity and enzymatic
product are not always either obvious or predictable for an unknown SMT. For
example, the plant SMT1
CA from algae and the protozoan SMT1
ZY can generate
similar 24-methyl-Δ
25(27)-olefins as the major product. Alternatively, the fungal
SMT1ZY from yeast and the plant SMT1
CA from soybean can generate similar 24
(28)-methyl(ene)-Δ
24(28)-olefins. The first C
1-activity of plant SMT2 catalyzes
cycloartenol to a single methylated product whereas the second C
1-activity catalyzes
24(28)-methylene lophenol to three methylated products. The ability of the
plant SMT2 to catalyze substrates of different features to different product sets
was shown to result from the specificity in molecular recognition of the Δ
24-sterol structure. Kinetically, the SMT1 from fungi and plants differ. In the case of the
yeast SMT1, the mechanism is random and for the plant SMT1 ordered so that
AdoMet is the leading substrate and must bind before sterol to the enzyme
(Nes, 2000; Nes
et al., 2003). Most likely, the different kinetic mechanisms relate
to the significance that the plant SMT1 has activities for both the first and second
C
1-transfer reaction and an ordered mechanism enables sequential C
1-transfer of
different acceptors in the active site.
SMT1 and SMT2 activities vary with respect to one another in plants
(Nes, 2000; Nes
et al., 1989b; Wentzinger
et al., 2002). For example, the proportion
of SMT1 to SMT2 activity measured as their catalytic competence (V
max/K
m) is
similar in cultured cells of tobacco during active cell proliferation. Alternatively,
as the plant matures and cell proliferation is arrested, the ratio of the two activities
change dramatically and can favor the activity of the second C
1-transfer reaction.
These findings are in agreement with the suggestion of Benveniste and coworkers
(Arnqvist
et al., 2003; Benveniste, 2004) of the possible importance of SMT2 as a
branch point enzyme in phytosterol synthesis. The activity of SMT1 to control
carbon flux into the phytosterol pathway and to cholesterol (Diener
et al., 2000;
Parker and Nes, 1992) is evidenced in the high specific activity of cholesterol
synthesis in mature leaves (Nes, 1990) and in potato plants overexpressing
a foreign SMT1 (Fonteneau
et al., 1977). Given the regulatory role of SMT1
and SMT2 to balance the ratio of C-8 to C-9 to C-10 sterols suggests that the
induced change in the ratio of SMT1 to SMT2 activities during development
is largely controlled at the level of transcription. However, effectors, such as
sitosterol or ATP, may also increase or decrease carbon flux through a branch
point in phytosterol synthesis by modulating SMT activity (Nes, 2000).
AdoMet-dependent methylation has been found to be the target of functional
convergence (Schubert
et al., 2003) generating a subfamily of SMTs that clearly
evolved their substrate specificity for sterol independently of related AdoMetdependent
methyltransferases. Genetic and bioinformatic research of the derived
amino acid sequences of over 60 SMTs deposited in the GenBank and other
databases, indicate the primary structure encodes about 380 (±20) amino acid
long proteins with an amino acid sequence relatedness which allows subdivision
of the SMT gene family into five subfamilies, designated SMT
a through SMT
e,
each distinguished by sharing a minimum of 40% identity among members
(Fig. 9.17). Certain sequence motifs shared by all SMTs suggest that they share a
common evolutionary origin. For example, three conserved domains in the primary
structure of SMTs, which we have previously designated as Regions I, II,
and III, are hydrophobic and always found in the same order on the polypeptide
chain and are separated by comparable intervals (Fig. 9.18). Chemical affinity
labeling and site-directed mutagenesis experiments have shown that Regions
I and III correspond to a sterol binding site and in the yeast SMT maps to
Y81EYGWGSSFHF and Y192AIEATCHAP (Nes
et al., 1999; Sinha, 2004; Zhou
and Nes, 2003). Photoaffinity labeling and site-directed mutagenesis experiments
show that Region II corresponds to a AdoMet binding site and in the yeast SMT
maps to L24DVGCGVGGP (Schaeffer
et al., 2000). That these enzymes are so
similar in much of their primary and presumably three-dimensional structures in spite of taxonomic differences in the species from which they are derived
suggests that they arose by divergent evolution from an ancestral gene prior to
the origin of eukaryotes.
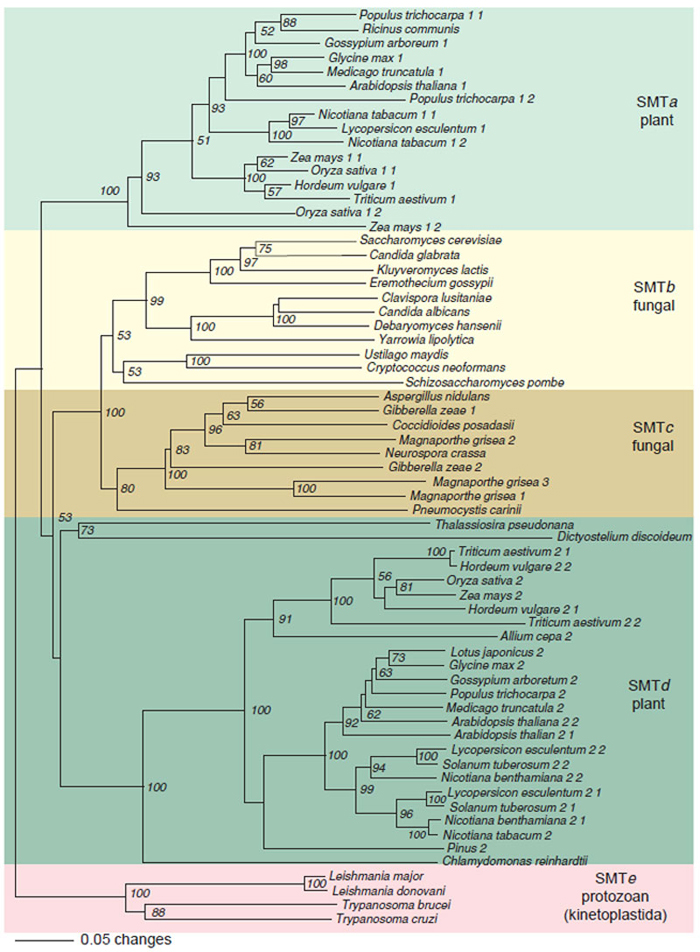 |
| FIGURE 9.17 Rooted phylogenetic tree of eukaryotic SMT was created with PAUP using the
neighbor-joining method with kinetoplastida SMTs as the out group. The scale bar represents a
distance of 0.05 substitutions per site. Numbers are the percentage bootstrap values for 1,000
replicates. SMTa through SMTe designate SMT subfamilies defined by a minimum of 35% identity
between members at the amino acid level. Accession number of SMT sequences obtained from
NCBI (http://www.ncbi.nlm.nih.gov): Ricinus communis, T10173; Glycine max 1, T06780; Arabidopsisthaliana 1, AGG28462; Nicotiana tabacum 1-1, AAC43951; Nicotiana tabacum 1-2, AAC35787; Zea mays 1-1, TO4138; Oryza sativa 1-1, AAC34988; Triticum aestivum 1, ABB49388; Oryza sativa 1-2,
AAP21419; Saccharmyces cerevisiae, NP_013706; Candida glabrata, CAG59930; Kluyveromyces lactis, AAS52116; Clavispora lusitaniae, CAO21936; Candida albicans, O74198; Debaryomyces hansenii, CAG87427; Yarrowia lipolytica, CAG77980; Ustilago maydis, EAK84412;
Schizosaccharomyces pombe, CAB16897; Gibberella zeae 1, XP_382959; Magnaporthe grisa 2,
EAA48309; Neurospora crassa, CAB97289; Gibberella zeae 2, XP_355916; Magnaporthe grisa 3,
EAA50587; Magnaporthe grisa 1, EAA47049; Pneumocystis carinii, AKK54439; Oryza sativa 2,
ACC34989; Arabidopsis thaliana 2-1, ABB62809; Arabidopsis thaliana 2-2, CAA61966; Nicotiana
tabacum, TO3848; Leishmania donovani, AAR92098; Trypanosoma cruzi, TIGR_5693. Gene indices
of SMT obtained from TIGR (http://www.tigr.org): Populus trichocarpa 1-1, TC35619; Medicago
trucatula, TC86500; Gassypium arboretum 1, TC20798; Populus trichocarpa 1-1, TC36329; Hordeum
vulgare 1, TC 110279; Zea mays 1-2, TC234797; Cryptococcus neoformans, TC4573; Aspergillus
nidulans, TC6295; Coccidioides posadasii, TC4513; Triticum aestivum 2-1, TC165856; Hordeum
vulgare 2–2, TC121611; Zea mays, TC224796; Hordeum vulgare 2-1, TC123636; Triticum aestivum 2-2,
TC172448; Allium cape 2, TC2207; Lotus japonicus, TC7995; Glycine max 2, TC189052; Gossypium arboretum 2, TC 21049; Populus trichocarpa 2, TC36329; Medicago truncatula 2,
TC77751; Lycopersicon esculentum 2-2, TC126730; Solanum tuberosum 2-2, TC126449; Nicotiana
benthamiana 2-2, TC7251; Lycopersicon esculentum 2-1, TC124648; Solanum tuberosum 2-1,
TC112127; Nicotiana benthamiana 2-1, TC8230; Pinus 2, TC52326; Chlamydomonas reinhardtii,
TC29837. Gene identification number of SMT from The Wellcome Trust Sanger Institute
(http://www.sanger.ac.uk): Leishmanis major, LM3731Bb05.p1c; Trypanosoma brucei, TB10.1520. The
SMT gene of Dictyostelium discoideum was from IMB (http://genome.imb-jena.de) and gene
identification number is pcr25kl1p3887. SMT gene of Thalassiosira pseudonana is identified from
GRI (http://genome.jgi-psf.org) in scaffold_30, 94659:96009. |
 |
| FIGURE 9.18 Alignment of representative
deduced amino acid sequences of SMT from
fungi,
protozoa, and plants that represent SMT1
and SMT2 isoforms. Conserved regions
corresponding to
sterol (Regions I and III) and
AdoMet binding sites (Region II) are boxed. |
To understand further the importance of conserved amino acids in the primary
structure and the molecular interactions between sterol and enzyme to correctly
position and discriminate the nucleophile that serves to undergo C-methylation to a
single product, mutagenesis and activity assay with carefully designed mechanismbased
inactivators were undertaken of highly conserved amino acid residues in
the SMT from yeast (Fig. 9.19). We have reported that several mutants of yeast
SMT modified in Region I, D79L and Y81F, produce mixtures of Δ
24(28)- and Δ
25(27)-
olefins depending on the nature of Δ
24-substrate (Nes
et al., 1999; Sinha, 2004; Zhou
and Nes, 2003). The yeast SMT1 can become plant-like in accepting Δ
24(28)-substrates
by a single mutation in Region I at Y81 (Nes
et al., 1999). The suicide substrate,
26,27-dehydrozymosterol, assayed with wild-type enzyme produces a novel
Δ
23-sterol with an elongated side-chain (Parker and Nes, 1992). The
steric–electric plug model permits differences in product specificity among SMT enzymes to have
arisen through point mutations which change either the shape of the catalytic site
and/or positions of the
 |
| FIGURE 9.19 Catalytic competence of native
and mutant yeast SMTs tested with
different
substrates. |
crucial functional groups. The D79L and Y81F mutants are
capable of overcoming the topological impediment to generate multiple products,
suggesting similar binding segments in the active center are present in all SMTs.
Testing with different sterol substrates of either heterologously expressed or
wild-type enzymes indicates that substrate specificities have evolved differently
in plants compared to the fungi and protozoa. All plant SMT1s reveal a strict
substrate specificity accepting cycloartenol whereas the fungal SMT1 and protozoan
SMT1 accept either zymosterol or lanosterol. A major difference between zymosterol and lanosterol is the presence of the geminal methyl group at C-4 in
the lanosterol structure. The 4,4-dimethyl group can sterically interfere with the
hydrogen bonding ability of the C
3-hydroxyl group thereby affecting substrate
affinity. Whereas SMT1 enzymes can be distinguished on their recognition of
the nucleus structure, SMT1 and SMT2 enzymes can be distinguished on their
recognition of the side-chain functional group Δ
24(25)- versus Δ
24(28)-substrate. To
account for the different substrate acceptability, different arrangements of similar
amino acid residues may have evolved in the active site of SMTs to interact with
the nucleophilic groups at C-3 and C-24.
No conclusive discussion for the order of SMT evolution could be drawn from
the unrooted phyogenetic tree (Fig. 9.17), based on the order of intermediates and
the positioning of SMT isoforms in phytosterol synthesis. However, plant SMT2s
which utilize 24(28)-methylene lophenol as the preferred substrate, but maintain
vestige substrate specificity for cycloartenol, must have evolved from a progenitor
plant SMT1 perhaps by duplication followed by mutation and divergence. An
important question that warrants further study is whether cycloartenol served as a
template providing functional constraints in the design and genetic formation of
the plant SMT active site during either evolution or random mutation with natural
selection provided the guiding principle of enzyme redesign.
A similarity in function (catalytic competence) among SMTs is reflected in the
enzyme ability to catalyze a sterol acceptor to a methylated product. However,
enzyme function of individual SMT isoforms in either the same plant or those
formed in different phyla can be different in terms of substrate acceptability,
kinetics, product outcome, and selectivity to inhibitors or effectors of SMT action.
Although it may be possible to use the conserved Regions I and II of cDNA
corresponding to SMT for homology-based cloning strategies, there is insufficient
information for predictions of catalysis of new SMTs that might be cloned from,
for example prokaryotes, since none of the putative prokaryote SMTs in the
GenBank contain Region I. The trace level of phytosterol in cyanobacteria, suggesting
they are contaminants (Marshall, 2007), and the likelihood SMTs are not
synthesized by photosynthetic bacteria make the timing of SMT production and
hence phytosterol accumulation during the course of evolution unclear.











