Pathways of Phytosterol Biosynthesis
The pathway to isoprenoids (five carbon units similar to isoprene) and phytosterols
in plants begins with CO
2 fixation and sugar formation (Fig. 9.3). Sugar can be
converted to acetate which can be converted to isoprenoids and phytosterols in
the cytosol. In producing phytosterols, acetate is converted to mevalonic acid
(MVA). The MVA is phosphorylated and the carboxyl carbon is lost as CO
2 to
produce Δ
3-isopentyl diphosphate (IPP). However, the sugar can be converted
to isoprenoids in the plastids without necessarily involving the intermediacy
of acetate via the mevalonate-independent pathway [=1-deoxy xylulose-5-
phosphate (DXP) pathway]. The DXP synthase is considered rate-limiting in this
pathway. Carbons from Δ
3-IPP can flow to the sterol or fatty acid pathways (via
the MVA shunt) (Nes and Bach, 1985). HMGCoA-reductase (HMGR) is considered
to be the rate-limiting enzyme of the acetate–mevalonate pathway to isoprenoids.
Six of the isoprenoid units are joined to produce squalene. The C-30
olefin is converted to squalene oxide which is cyclized to 24-desalkyl sterols. The
extra ‘‘methyl or ethyl’’ group at C-24 in the side-chain of phytosterols is added after formation of the first tetracycle by the action of SMT. In the phytosterol
pathway, SMT is considered to be a rate-limiting enzyme.
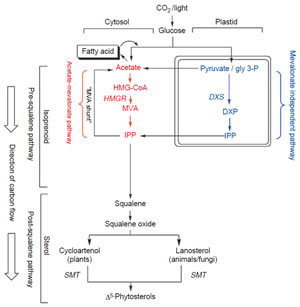 |
| FIGURE 9.3 Stages in the
isoprenoid–phytosterol pathway and
compartmentation of
acetate–mevalonate
pathways. |
Biosynthetic tracer studies have been carried out on almost all classes of
phytosterols, and in many instances cell-free preparations capable of converting
Δ
24-sterols to methylated products are available. In almost all instances, the
C-methylation processes are consistent with an ionic mechanism hypothesized
by Castle et al. (1963), attesting to a common set of reactions that evolved for this
class of enzyme. Detailed information about isoprenoid-sterol biosynthesis and the
pathways involved in sterol side-chain construction has been obtained recently
using
13C and
2H-labeled compounds (Kresge
et al., 2005; Seo
et al., 1988, 1990).
Isotopic labeling experiments with stable isotopes are often used to avoid radioactive
measurements and laborious chemical degradation of the sterol side-chain
(Nes and Le, 1990). The sites of labeling in the end product are evident through
peak enhancements in the
13C NMR spectra of the
13C-labeled sterols and reveal
the route of carbon flux from the isoprenoid pathway to phytosterols.
Plant biochemists have shown that two distinct pathways to sterols exist in
the cell and they are compartmentalized so that the acetate–mevalonate pathway
is cytosolic and the mevalonate-independent pathway is plastidial (Arigoni
et al., 1997; De-Eknamkul and Potduang, 2003; Laule
et al., 2003; Lichtenthaler
et al., 1997; Nes
et al., 1992; Umlauf
et al., 2004; Zhou and Nes, 2000). Some typical
experiments to determine the pathway of phytosterol synthesis involve treating
plants with [1-
13C]glucose. The sugar is converted to [3-
13C]pyruvate which is
converted to [2-
13C]acetate and the C-2 unit is converted in several reactions to
[2,4,5-
13C
3]Δ
3 IPP; or the [3-
13C]pyruvate is converted to [3-
13C]glyceraldehyde
3-phosphate and this intermediate is transformed to [1,5-
13C
2]Δ
3-IPP. The labeling
pattern of, for example, ergosterol derived from administering cells [1-
13C]glucose
has been found to be different in different organisms whether the Δ
3-IPP is formed
from the glucose breakdown product of [2-
13C]acetate or originates directly from
the intermediate DXP (Zhou and Nes, 2000; Lichtenthaler
et al., 1997). When
the plastid-derived intermediates are labeled with [1-
13C]glucose, the labeling
pattern of the
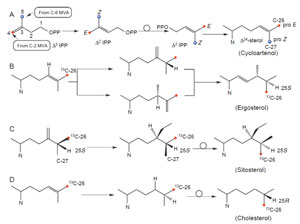 |
| FIGURE 9.4 Determination of labeling patterns in
isopentyl diphosphate and sterols synthesized
by
different pathways. |
biosynthetically formed sterol molecule is predicted to be
[2,6,11,12,16,18,19,23,27-
13C
9]ergosterol. A spectrum of ergosterol, biosynthesized
from [1-
13C]glucose using the alga
Prototheca wickerhamii shows enhanced peaks
corresponding to nine carbon atoms (earlier we incorrectly identified the
enhanced peak corresponding to C-11 and C-21 to be from [1-
13C]glucose (Zhou
and Nes, 2000); actually the enhanced peak is due to C-11 only; Fig. 9.5; spectrum
c), suggesting the alga operates the mevalonate-independent pathway to sterols.
Alternatively, the labeling pattern of the products of the acetate-mevalonate pathway
is predicted to be [1,3,5,7,9,13,15,17,18,19,21,22,24,26,27-
13C
15]sitosterol (De-
Eknamkul and Potduang, 2003;Umlauf
et al., 2004). Since neither C-28 nor C-29 will
be labeled by these intermediates, the labeling patterns of ergosterol and sitosterol
assayed with [1-
13C]glucose will be the same (Fig. 9.4). Evidence to confirm the
traditional isoprenoid–sterol pathway was obtained by administering [2-
13C] MVA
or [5-
13C]MVAto culturedsunflower cells andnotingthenumber andpositionof the
enhanced peaks in the
13CNMR spectrum(Fig. 9.5; spectra a and b) (Nes
et al., 1992).
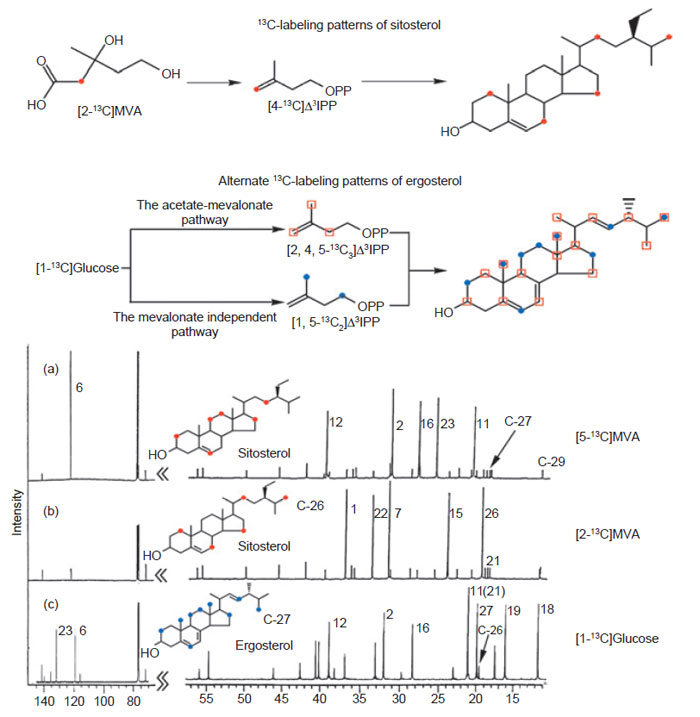 |
| FIGURE 9.5 Stereochemistry of phytosterols at C-25 after 13C labeling of the ProE C-26 of
Δ24-sterols. |
As shown in Fig. 9.5, C-26 and C-27 of the sterol side-chain are chemically
equivalent yet they are biosynthetically distinct. Seo et al. (1990) have shown that
C-26 is derived from C-6 of MVA and C-27 is derived from C-2 of MVA. Appropriate
rotations in the structures of these compounds generate a view of the sterol
side-chain in equivalent conformation so that the stereochemistry at C-25 can be
rationalized and the existence of stereodifferentiated enzymes determined from
tracer studies with
13C-labeled substrates. Nes et al. (1992) examined the incorporation
of [2-
13C]MVA to sitosterol and found that C-26 is labeled and Seo et al.
labeled C-26 in ergosterol with [2-
13CH
3]acetate (Zhou and Nes, 2000). To confirm
that the stereochemistry at C-25 is the same in sitosterol and ergosterol,
acceptors [27-
13C]zymosterol, [27-
13C]lanosterol, and [27-
13C]cycloartenol were
prepared and assayed with cell-free preparations from plants, fungi, and algae
(Guo
et al., 1996; Mangla and Nes, 2000; Nes
et al., 1998b; Zhou
et al., 1996).
The enzymatically formed side-chains of fecosterol, 24(28)-methylene lanosterol,
and cyclolaudenol contained the C-25R-configuration as determined by 13C NMR spectroscopic analysis, thereby showing the ProZ C-27 of the Δ
24-intermediate
generates the R-C-27 methyl group of the phytosterol.
Incubation of [1-
13C]glucose with cultured cells or intact plants can generate
different
13C-labeled species of phytosterol and the purity of the labeling pattern
of the sterol can indicate the degree to which the acetate–mevalonate pathway or
mevalonate-independent pathway operates and the extent of cross talk between the
pathways (Arigoni
et al., 1997; Laule
et al., 2003). These studies indicate that the
acetate–mevalonate pathway (=isoprenoid pathway) is preferred in vascular plants.
This observation is further substantiated by physiology experiments with inhibitors
of HMGR such as mevinolin added to radish seedlings (Bach and Lichtenthaler,
1983) or fosmidomycin, an inhibitor of 1-deoxy-D-xylulose-5-phosphate reductoisomerase,
added to tobacco cells (Sauvaire
et al., 1997), and by flux studies using
tracer amounts of radiolabeled acetate and high concentrations of mevalonate to
inhibit sterol synthesis in sorghum seedlings (Hemmerlin
et al., 2003).
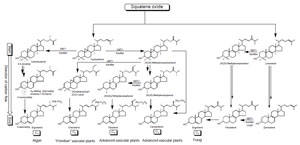 |
| FIGURE 9.6 Hypothetical pathways to
Δ5-phytosterols: C1 and C2 refer to the CH3
and C2H5 groups attached to C-24 of the sterol
side-chain. Circled
C-4 is to emphasize C-4
methyl group removal; 4,4-dimethyl sterol to
4-monomethyl sterol to 4-desmethyl sterol. |
In the past several years, there have been several remarkable advances in the
study of phytosterol enzymes to support the hypothesis that multiple phytosterol
pathways exist in nature. The start and direction of the pathway is established by
whether squalene oxide cyclizes to cycloartenol (plants and algae) or lanosterol
(animals and fungi) (Fig. 9.6; Nes and McKean, 1977). Thereafter, the C-24 methylation
pathways provide direction to phytosterol synthesis; ‘‘primitive’’
plants catalyze the Δ
25(27)-route whereas advanced plants and fungi catalyze the Δ
24(28)-route (Goad
et al., 1974; Nes
et al., 1977). Mass spectroscopy (MS) can be
used as a first screen to determine whether ergosterol is formed by either a fungal
or algal route. For example, administering [
2H
3]methionine to a yeast sterol
auxotroph GL7 cultured on lanosterol led to the biosynthesis of [28-
2H]ergosterol
(Zhou
et al., 1996). The mass spectrum of the deuterated ergosterol was found to
be two mass units higher than the control species, consistent with methylation
at C-24 proceeding by a Δ
24(28)-methylene intermediate. The site of introduction
of methyl in the sterol side-chain was determined by inspection of the
1H NMR
spectra of ergosterol
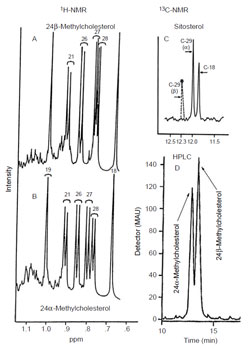 |
| FIGURE 9.7 Spectroscopic and
chromatographic analysis of sterols. |
and the corresponding deuterium-labeled ergosterol
(Fig. 9.7, Panels B and A). The only signal in the
1H NMR spectrum affected by
the incorporation of deuterium in the molecule corresponds to C-28 which is
lowered compared to the control. In related experiments with a cell-free preparation
of SMT from yeast assayed with [
2H
3-
methyl]AdoMet and zymosterol, the
enzymatic product [28-
2H]fecosterol contained two extra deuterium atoms as determined by MS; in the
1H NMR spectrum, the olefin peak at ca. 4.65 ppm (not
shown) corresponding to C-28 was missing but it was present in the spectrum of
unlabeled fecosterol (Fig. 9.7; Nes
et al., 1998b). These findings show methyl from
AdoMet is added to C-24 of the sterol side-chain and becomes C-28 of yeast
ergosterol via a pathway that involves methylation of zymosterol to produce the
Δ
24(28)-olefin fecosterol, as expected (Goad
et al., 1974). In the pathway to algal
ergosterol, activity assay with [
2H
3-
methyl]AdoMet and cycloartenol produces an
intermediate molecule cyclolaudenol that mass spectroscopy reveals contains three
rather than two deuterium atoms (Zhou
et al., 1996). Thus, the methylation route to
fungal ergosterol proceeds by a Δ
24(28)-route whereas the algal route proceeds by
the D25(27)-route.
Phytosterols will incorporate either four (α-configuration) or five (β-configuration)
deuterium atoms in the 24-ethyl group after treatment with [
2H
3-methionine]
(Goad
et al., 1974), but in these instances the sterols can be derived from the same
SMT by a similar C-24-methylation mechanism (Kaneshiro
et al., 2002; Nes
et al., 2003).
To address whether the mechanism of sterol C-methylation leading to
vascular plant campesterol (24α-methyl group) is the same as the one leading to
fungal ergosterol (24β-methyl group), Nes and coworkers recovered 27-
13C-24(28)-
methylene lanosterol from activity assay of 27-
13C-lanosterol prepared in a cellfree
corn system (Guo
et al., 1996). The
13C-labeled compound was administered to
a yeast sterol auxotroph GL7 which converted the dietary supplement to [27-
13C]
ergosterol (Zhou
et al., 1996). The
13C NMR spectrum of either the [27-
13C]ergosterol
derived by the plant- or fungal-generated [27-
13C]-labeled intermediates were
identical, indicating the phytosterol C-methylation pathway in the two organisms
is similar and involves a 1,2-hydride shift from the Re-face of the original substrate
undergoing methylation. Using cloned SMTs from a mutant yeast (Y81W) and
wild-type soybean and Arabidopsis, a single enzyme was found to catalyze both
the first and second C
1-transfer activities, and in the case of the second C
1-transfer
activity the stereochemistry of the 24-ethyl group (β) in the product was shown
to be opposite to that of the 24-ethyl group in sitosterol (α) (Fig. 9.6). Inevitably, the
conclusion that different phytosterol pathways are present in plants and fungi
stems from the fact that different enzymes control the stereochemistry of the
methylated products and that the substrate affinities and catalytic competence of
SMTs can be different in different organisms.









