Introduction
The vast majority of naturally occurring sterols contain an alkyl group at carbon-24
in the side-chain. 24-Alkyl sterols are collectively knownas phytosterols and possess
chemical structures similar to that of cholesterol (cholest-5-en-3β-ol). Sitosterol is the
major C
29-Δ
5-phytosterol and ergosterol is the major C
28-Δ
5-phytosterol of plants
and fungi, respectively. Cholesterol is the major C
27-Δ
5-zoosterol of animals and
insects (Fig. 9.1). Pure sitosterol resembles cholesterol, white in color and waxy
in nature. The physical resemblance of the two sterols is mirrored in the
similarities of the biosynthetic pathways and functions of these compounds. A
striking feature of phytosterol synthesis is that the pathway is directed to form
membrane components, in a similar fashion as for cholesterol production and
processing in animal systems. However, the subtle differences in the structures of
the sterol side chains, 24-methyl (or ethyl) group compared to a 24-hydrogen atom,
make for plant-specific functions of sterols.
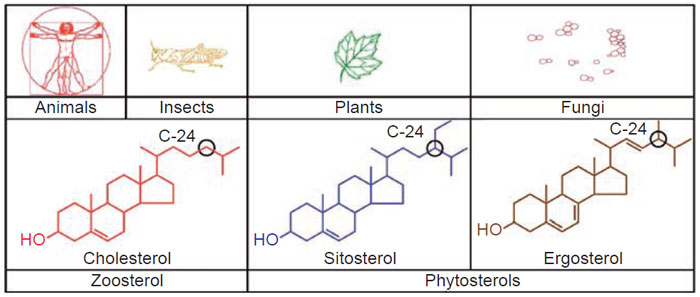 |
| FIGURE 9.1 Major sterols found in living systems. |
Phytosterolsmake up greater than 80%of the total sterol content of the vegetative
parts of plants and accumulate to 500–3,000 fg/cell or 1 mg/blade, mostly as free
sterol (Nes, 1990; Nes and McKean, 1977). High phytosterol levels are found esterified
to fatty acids in seed oils and in leaves of aging plants (Nes, 1990;
Wojciechowski, 1991). In humans, cholesterol is present in cells about 500–
3,000 fg/cell, similar to the sterol content of plant cells, and about 10 mg/liter in
the serum (Nes, 1990; Nes
et al., 2000). Alternatively, under normal physiological
conditions phytosterols are found
at 800–1,000 times lower concentration than that
of endogenous cholesterol in the serum. When the concentration of phytosterol is
maintained at a high level, ca. 300 mg up to 5 g/day, by a diet rich in vegetables and
fruits or through vegetable oil spreads, such as Benecol or Take Control, there are
important benefits. For example, phytosterols have been shown experimentally to
inhibit colon, breast, and prostate cancers; induce anti-inflammatory effects; and
reduce cholesterol levels (Awad and Fink, 2000; Moreau
et al., 2002; Tapiero
et al., 2003). The exact mechanism by which sitosterol offers protection from cancer is not
known and several theories have been advanced as reviewed (Ling and Jones, 1995). On the other hand, it is generally assumed that cholesterol reduction in the blood
stream results directly frominhibition of food-based cholesterol absorption through
displacement of cholesterol from micelles (Akihisa
et al., 2000; Moreau
et al., 2002).
For these reasons, phytosterols are considered nutraceuticals and the engineering of
plants to increase the phytosterol content as a nonpharmacological approach to
prevent certain diseases is underway. It is worth pointing out that there is a portion
of the literature that continues to use beta (β)-sitosterol; the beta in this case does not
refer to the stereochemistry of the molecule but is used to distinguish the compound
from α- and γ-sitosterol. The notation is dropped in common usage (Nes, 2000).
In contrast to other plant lipids, such as fatty acids defined on the basis of their
physical properties, phytosterols are defined by their common chemical structure
and biosynthetic reasoning related to the cyclization of squalene oxide (Nes and
McKean, 1977). Phytosterols, which are insoluble in water and can be extracted
from cells by nonpolar organic solvents (such as hexane or chloroform), are characterized
by a cyclopentanoperhydrophenanthrene structured nucleus, a flexible
side-chain of 9 or 10 carbon atoms and equatorial attachments of a polar head
(3β-hydroxyl group) and nonpolar tail (17β-side-chain). The three-dimensional
shape is established by the alternating all
trans–anti stereochemistry of the ring
system and the 20R-configuration that directs the side-chain to a ‘‘right-handed’’
conformation (Nes
et al., 1984; Parker and Nes, 1992; Fig. 9.2). When the side-chain
of, for example, sitosterol is oriented to the ‘‘right,’’ the sterol has the appropriate length—ca. 19Å to fit the monolayer—that is, one-half of the lipid leaflet
structure. The combination of asymmetry and electronics in the sitosterol
structure gives rise to an amphipathic molecule, basically flat with a length suitable
for the sterol to insert the membrane. The presence of 24-ethyl sterols in plant
membranes correlates with their efficiency in developing interactions with
plant phospholipids to affect membrane fluidity (Mckersie and Thompson, 1979;
Schuler
et al., 1991).
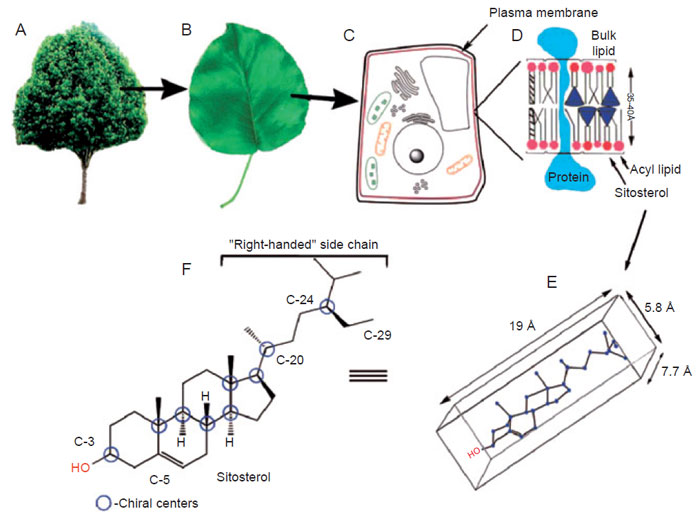 |
| FIGURE 9.2 Sitosterol distribution in the intact plant: (A) whole plant; (B) leaf; (C) cell structure;
(D) membrane lipid leaflet; (E) conformational perspective of sterol; (F) structure and
stereochemistry in sitosterol. |
The C-24 alkylated family of phytosterols contains a greater number of
individual compounds in plants than fungi or protozoa. The variant structures
and health benefits of generating a modified phytosterol composition provide
the basis for investigations of phytosterol profiling and metabolic engineering.
Studies on testing inhibitors of sterol methyltransferase (SMT) action in cultured
cells and genetic manipulation of the phytosterol pathway in several plants have
revealed a physiological requirement for a 24-alkyl substituted steroid in plant
growth, the central position of C-methylation in the plant sterol pathway and the
connection of the SMT to manufacturing value-added traits (Chappell
et al., 1995;
Harker
et al., 2003; Nes
et al., 1991c; Rahier
et al., 1980). Since the biosynthesis and
functions of sterols in plants are covered in recent review articles (Benveniste,
2004; Clouse, 2002; Lindsey
et al., 2003; Nes, 2003; Schaller, 2004), we have
included only background material pertinent to the phytosterol pathway as
influenced by the SMT. Initially, the pathways of sterol biosynthesis will be
described. After a brief coverage of phytosterolomics, with emphasis on structure
determination, we review the enzymology and evolution of the SMT and
conclude with the current state of metabolic engineering sterol pathways in
plants. We have not attempted to be comprehensive in our presentation of the
literature; rather the focus is on illustrative examples that may serve to indicate
future opportunities to engineer plants with unusual sterol profiles or traits.






