Cell Culture and Metabolite Production
When we cultivate plant cells
In vitro, there are different types of cultures with
distinct metabolite productivities. One type produces metabolites in undifferentiated
cells cultured
In vitro, while another produces metabolites only under differentiated
conditions, for example, shoot or root cultures. In some cases, cells lose their biosynthetic potential even after redifferentiation, that is, showing the
complete loss of their natural biosynthetic potential.
The first type of cell culture is in high demand because cells are easily
cultivated under
In vitro conditions. Plant cells often do not produce desired
secondary metabolites, although they have the potential to regenerate whole
plants from single cells. For example, plant growth regulators, such as auxins
and cytokinins, can regulate morphological differentiation, but the chemical
regulation of functional differentiation in secondary metabolism without morphological
differentiation is rather limited. For example, morphinan alkaloids are not
produced in cell cultures of
P. somniferum without organ differentiation (Facchini
and Park, 2003; Grothe
et al., 2001; Huang and Kutchan, 2000; Unterlinner
et al., 1999), whereas several cell cultures, for example,
P. somniferum and
C. japonica,
produce large quantities of structurally related isoquinoline alkaloids, sanguinarine
and berberine, respectively (Facchini and Park, 2003; Huang and Kutchan,
2000; Sato and Yamada, 1984). Interestingly, morphine, sanguinarine, and
berberine are derived from tyrosine through the same intermediate, reticuline
(Fig. 11.2), indicating that early steps in metabolic pathway do not determine the
end-product in cell culture.
Biochemical, molecular biological, and cell biological studies of biosynthetic
enzymes have gradually revealed the mechanisms of regulation. For example, all
enzymes examined were highly expressed in cultured
C. japonica cells, which
show a high production of berberine (Ikezawa
et al., 2003; Sato
et al., unpublished
data), and in
P. somniferum cells, which do not produce morphinan alkaloids
under undifferentiated conditions (Facchini and Park, 2003; Grothe
et al., 2001;
Huang and Kutchan, 2000; Unterlinner
et al., 1999). However, biosynthetic
enzymes in sanguinarine biosynthesis in roots have been localized to the immature
endodermis and the protodermis of leaf primordia in the rhizome of
Thalictrum (Samanani
et al., 2005). Similarly, the enzymes in morphinan alkaloid
biosynthesis were localized in different cell types (see above); localization of
40OMT and SAT were both in phloem parenchyma cells, but the enzyme catalyzing
the penultimate step, COR, in morphine biosynthesis was located in the
laticifers (Kutchan, 2005a; Weid
et al., 2004). These results suggest that secondary
metabolite production in cell cultures is regulated in a complicated manner. The
coordinated expression of biosynthetic genes and their enzymes at a high level
seems to be an essential requirement for high production of metabolites.
Many medicinal compounds are used as chemical defense agents in whole
plants. These metabolites often have activities as phytoalexins which are induced
in response to fungal attack, that is, to act as endogenous chemical weapons for
defense in plants. For example, berberine and sanguinarine are antibacterial and
antifungal agents (Schmeller
et al., 1997). These chemicals are also produced in
cells/tissues cultured
In vitro, even though they are cultivated under aseptic conditions
without infection by microbes or attack by animals. The high expression of
pathogenesis-related protein genes, as well as of cell proliferation-related genes in
cultured cells, has been indicated by protein analysis and expressed sequence tag
(EST)- and microarray analyses, indicating that the cell cultures exhibit stress
responses (Sasaki
et al., 1994; Takeda
et al., 1990; Sato
et al., unpublished data).
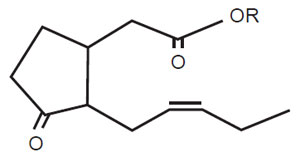 |
| FIGURE 11.5 Active jasmonic acid structure.
JA; R=H; MeJA, R=CH3. |
JA (or MeJA) (Fig. 11.5) is an active component in stress responses, especially in
elicitation induced by microbial cell walls, heavy metals, and so on (Gundlach
et al., 1992; Zhao
et al., 2005). Among signal mediators, JA has a pronounced effect in
the production of several secondary metabolites, including paclitaxel (Yukimune
et al., 1996). However, the application of JA alone is not sufficient to induce the
entire biosynthetic pathway for indole alkaloids (Eilert
et al., 1987; Van Der Fits and
Memelink, 2000): the application of JA induced only a set of biosynthetic genes
(Fig. 11.3). Some high-metabolite-producing cells do not respond to JA, suggesting
that the signal transduction system and/or the expression of some downstream
biosynthetic gene(s) may be highly activated during cell selection (Sato
et al., unpublished data). While many of the biosynthetic genes in secondary metabolism
respond to JA, PMT in tropane alkaloid synthesis does not (Suzuki
et al., 1999a).
Regarding defense responses, we have identified several mediators for such
signals other than JA, including salicylic acid (SA) and ethylene. Recent advances
in the molecular biology of signal transduction have shown that the overall
mechanism of regulation of the expression of defense genes is more complicated
and divergent among plant species than expected (Vom Endt
et al., 2002;
Zhao
et al., 2005): for example, the ‘‘ethylene response factor 1’’ in
Arabidopsis acts downstream of the intersection between the ethylene and JA pathways,
suggesting that these signals are somehow integrated (Lorenzo
et al., 2003; Vom
Endt
et al., 2002). Although a general antagonism between JA and SA and synergistic
interaction between JA and ethylene have been noted in plant–pathogen
interactions, it is still too early to make any definite conclusions about this topic.





