Tropane Alkaloid and Nicotine Biosynthesis
Tropane alkaloids are mainly found in the Solanaceae and include the anticholinergic
drugs atropine, hyoscyamine, and scopolamine and the narcotic cocaine.
N-methylputrescine, the central precursor in tropane alkaloid biosynthesis, is also
an intermediate in the nicotine pathway. N-methylputrescine is produced by the
decarboxylation of ornithine or arginine by ornithine decarboxylase (ODC)
or arginine decarboxylase (ADC), respectively. Tropane alkaloids and nicotine
biosynthesis are also closely related to polyamine metabolism (Fig. 11.4). The first
committed step in tropane/nicotine alkaloid biosynthesis is catalyzed by the
SAM-dependent putrescine N-methyltransferase (PMT) (Hibi
et al., 1994), which
is highly homologous to spermidine synthase. Methylputrescine is subsequently
deaminated by a diamine oxidase, and spontaneous cyclization then forms
the reactive N-methyl-Δ
1-pyrrolinium cation. The latter is thought to provide a
precursor of the tropane ring or nicotinic acid to form nicotine, although details
are not available. PMT in nicotine biosynthesis is expressed specifically in the
cortex and endodermis of tobacco root tips, whereas strong expression is seen in
the xylem parenchyma and outer cortex cells in more differentiated parts of the
root (Hibi
et al., 1994).
PMT genes have also been isolated from tropane alkaloid-producing
Hyoscymus niger (
HnPMT) and
Atropa belladonna (
AbPMT).PMT promoter and β-glucuronidase
(GUS) fusion gene showed that AbPMT is expressed specifically in root pericycle
cells (Suzuki
et al., 1999a). While tropane alkaloids and nicotine are mainly synthesized
in the root and transported to aerial partswhere they accumulate in vacuoles to
high levels, the biosynthesis of these alkaloids might nevertheless be differentially
regulated (see below).
Tropinone is located at a branch point in tropane alkaloid synthesis. Two related
dehydrogenases, tropinone reductase I (TR-I) and tropinone reductase II (TR-II),
stereospecifically reduce the 3-keto group of tropinone to the 3α- and 3β-groups
of tropine and ψ-tropine, respectively. cDNA clones for TR-I and TR-II have been
isolated from
Datura stramonium (Nakajima
et al., 1993). A further analysis of their
localization suggested that TR-I and TR-II were localized differently and might have
different functions (Nakajima and Hashimoto, 1999). Nortropane polyhydroxylated
alkaloids, calistegines, are also assumed to originate from ψ-tropine. They have been
isolated from different species in the Solanaceae (Scholl
et al.,
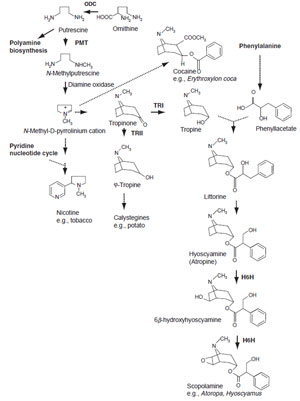 |
| FIGURE 11.4 Biosynthetic pathways to tropane
alkaloids, related compounds, and nicotine.
Unbroken arrows indicate single enzymatic
conversions and broken arrows indicate multiple
enzymatic steps. Enzymes for which the
corresponding genes have been cloned are
indicated in
bold. ODC, ornithine decarboxylase;
PMT, putrescine N-methyltransferase; TR-I/II,
tropinone
reductase I/II; H6H, hyoscyamine
6β-hydroxylase. |
2003, and references
cited therein). Calistegines showglycosidase-inhibiting activities and are considered
nutritional mediators in the rhizosphere (Tepfer
et al., 1988).
Hyoscyamine is produced by condensation of tropine and the phenylalaninederived
intermediate (R)-phenyllactate. Hyoscyamine can be
converted to its
epoxide scopolamine via 6β-hydroxylhyoscyamine by a 2-oxoglutarate-dependent
dioxygenase, hyoscyamine 6β-hydroxylase (H6H) (Matsuda
et al., 1991). H6H
localizes in the pericycle in branch roots of several scopolamine-producing Solanaceae
plants (Hashimoto
et al., 1991). Histochemical analysis using
H. niger and
A. belladonna H6H promoter::GUS fusion gene also showed that cell-specific
expression of the H6H gene is controlled by (unknown) genetic regulation specific
to scopolamine-producing plants but is absent in tobacco that does not produce
scopolamine (Kanegae
et al., 1994; Suzuki
et al., 1999b).
In
Nicotiana sylvestris, a set of nicotine biosynthesis genes was activated by the
exogenous application of methyl jasmonate (MeJA), but this activation was effectively
suppressed by simultaneous treatment with ethylene (Shoji
et al., 2000),
even though ethylene and JA are generally considered to act synergistically.
In contrast, treatment of
A. belladonna roots with MeJA did not lead to upregulated
expression of AbPMT genes (Suzuki
et al., 1999a). The different responses of
tropane alkaloids and nicotine biosynthesis to JA and ethylene suggest that
these biosyntheses might be under the control of different genetic regulation
systems (Shoji
et al., 2000).





