Terpenoid Indole Alkaloid Biosynthesis
Terpenoid indole alkaloids consist of about 3000 compounds, including the antineoplastic
vinblastine fromMadagascar periwinkle (
Catharanthus roseus), camptothecin
from
Camptotheca acuminata, and the antimalarial quinine from
Cinchona spp. The
central intermediate in the biosynthesis of terpenoid indole alkaloids is strictosidine,
which is produced from tryptamine and the iridoid glucoside secologanin by STR
(Fig. 11.3). Tryptamine is produced by tryptophan decarboxylase (TDC). While a
single gene in
C. roseus responds in both developmental and inducible expression
(De Luca
et al., 1989; Goddijn
et al., 1992), two genes in
C. acuminata showed different
expression profiles, suggesting that one is involved in developmentally controlled
camptothecin production in shoot apex and bark, while the other is involved in an
inducible defense mechanism (Lopez-Meyer and Nessler, 1997).
Two steps in secologanin biosynthesis are catalyzed by P450-dependent
enzymes: geraniol 10-hydroxylase (G10H) converts geraniol to 10-hydroxygeraniol
(Collu
et al., 2001), while secologanin synthase (CYP72A1) converts loganin to
secologanin and showsepidermis-specific expression in immature leaves of
C. roseus (Irmler
et al., 2000). The supply of terpenoid precursors should be rate-limiting in terpenoid indole alkaloid biosynthesis. The addition of secologanin or loganin to
C. roseus cell culture increases alkaloid accumulation (Whitmer
et al., 1998), and the
level of G10Hactivity is also positively correlated with the accumulation of alkaloids
(Facchini, 2001).However, secologanin is inefficiently used in strictosidine synthesis
when added to the medium since exogenous secologanin appears to be compartmentalized
differently from endogenous secologanin. This result also suggests that
the proper subcellular localization of biosynthetic enzymes and substrates is important
for the efficient biosynthesis of metabolites. These isoprenoid precursors are
also known to be derived from a nonmevalonate pathway (Contin
et al., 1998).
STR is a key enzyme in terpenoid indole alkaloid biosynthesis and cDNAs
have been isolated from
Rauvolfia serpentina (Kutchan
et al., 1988) and
C. roseus (Mcknight
et al., 1990). STR is one of the most investigated biosynthetic genes in
secondary metabolism. Strictosidine is deglucosylated by strictosidine glucosidase
(SGD) (Geerlings
et al., 2000) and then converted via several unstable
intermediates. While there is limited information available on the pathway to
catharanthine, vindoline biosynthesis has been relatively well characterized,
although the production of vindoline in cultured cells is limited.
The first of six steps in the conversion of tabersonine to vindoline consists
of hydroxylation at the C-16 position by tabersonine 16-hydroxylase (T16H), a
P450-dependent monooxygenase. While several P450 sequences
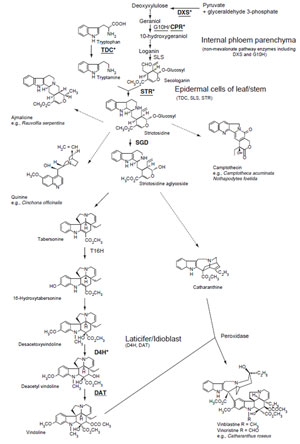 |
| FIGURE 11.3 Biosynthetic pathways to various
terpenoid indole alkaloids. Unbroken arrows
indicate single enzymatic conversions and dotted
arrows indicate multiple enzymatic steps.
Enzymes for which the corresponding genes have
been cloned are indicated. JA-inducible genes
are
indicated in bold. Underlining indicates that the
corresponding genes have been tested for
being
an ORCA3 target gene in C. roseus cells.
Enzyme-encoding genes regulated by ORCA3
are
asterisked (Vazquez-Flota et al., 2000).
DXS, D-1-deoxyxylulose 5-phosphate synthase;
STR,
strictosidine synthase; TDC, tryptophan
decarboxylase; G10H, geraniol 10-hydroxylase;
CYP72A1,
secologanin synthase; SGD,
strictosidine glucosidase; T16H, tabersonine
16-hydroxylase; CPR,
cytochrome P450
reductase; D4H, desacetoxyvindoline
4-hydroxylase; DAT, acetylcoenzyme
A: deacetylvindoline 4-O-acetyltransferase. |
were amplified
by polymerase chain reaction (PCR), the active principle of CYP71D12 was finally
identified to be T16H using translationally fused protein expressed in
Escherichia coli (Schroeder
et al., 1999). Interestingly, while
C. roseus has a single copy of the
TDC, STR, and cytochrome P450 reductase (CPR) genes, it has at least two T16H
genes. The 16-hydroxylation of tabersonine is followed by 16-O-methylation by a
cytosolic SAM: 16 hydroxyltabersonine O-methyltransferase (St-Pierre and
De Luca, 1995), hydration of the 2,3-double bond by an as yet uncharacterized
enzyme, and N-methylation of the indole-ring nitrogen by a thylakoid-associated
SAM: 2,3-dihydro-3-hydroxytabersonine-N-methyltransferase.
The penultimate step in vindoline biosynthesis is catalyzed by a cytosolic
2-oxoglutarate-dependent dioxygenase that hydroxylates the C-4 position of
desacetoxyvindoline 4-hydroxylase (D4H) (Vazquez-Flota
et al., 1997), and the
final step is catalyzed by the cytosolic acetylcoenzyme A: deacetylvindoline 4-Oacetyltransferase
(DAT) (St-Pierre
et al., 1998). The expression of T16H, D4H, and
DAT in developing
C. roseus seedlings is light regulated. Although D4H and DAT
activities are detected exclusively under conditions that result in vindoline biosynthesis,
T16H is expressed at low levels in
C. roseus cell cultures that do not
accumulate vindoline (St-Pierre and De Luca, 1995). The expression of D4H
appears to be under complex, multilevel developmental and light regulation.
A series of experiments with leaves of
C. roseus (Burlat
et al., 2004; St-Pierre
et al., 1999) showed that at least three cell types are involved in vindoline
biosynthesis.
The nonmevalonate pathway genes (DXS, 1-deoxy-D-xylulose
5-phosphate synthase, 1-deoxy-D-xylulose 5-phosphate reductoisomerase, and
2C-methyl-D-erythriotol 2,4-cyclodiphosphate synthase) as well as G10H were
found to be expressed in internal phloem parenchyma of the young aerial organs (Burlat
et al., 2004). Other early stage enzymes in the biosynthesis of strictosidine,
such as TDC, SLS, and STR, were expressed specifically in the upper and lower
epidermis of young leaves, stem, and flower buds. Late-stage enzymes in vindoline
biosynthesis, such as D4H and DAT, were localized in laticifer and idioblast cells,
which showgreater yellowautofluorescence with fewchloroplasts, compared to the
surrounding red-autofluorescent mesophyll cells. Light, which is not required for
the formation of these cell types, is required for activation of the localized expression
of the late stages of vindoline biosynthesis (Vazquez-Flota
et al., 2000).
Vindoline biosynthesis is restricted to the aboveground organs, and the pathway
beyond tabersonine is not expressed in tissue cultures (Vazquez-Flota
et al., 2002), whereas catharanthine accumulates in cultured cells as well as etiolated
seedlings.
These facts, along with the recovery of vindoline biosynthesis in regenerated
shoots, suggest that the biosynthesis of catharanthine and vindoline is
differentially regulated and that vindoline biosynthesis is under more rigid tissue-,
developmental-, and environmental-specific control than that of catharanthine
(St-Pierre
et al., 1999). These results raise the possibility that these cell
cultures lack the cell types required to accommodate the late stages of vindoline
biosynthesis.
Until recently, characterization of terpenoid indole alkaloid biosynthesis has
mainly been carried out with
C. roseus, but the recent establishment of hairy root
cultures of
Ophiorrhiza pumila (Rubiaceae) that showed high camptothecin production
provided another useful experimental system. Computer-aided atomic reconstruction
of metabolism and tracer experiments with [1–
13C] glucose indicated that
camptothecin is formed by the combined activities of the 2C-methyl-D-erythritol
4-phosphate pathway and the shikimate pathway (Yamazaki
et al., 2004).





