Organ Differentiation and Secondary Plant Products
For the second case where metabolites are produced during organ differentiation,
organ culture is a suitable alternative even on a large scale (Curtis, 1993; Kusakari
et al., 2000). Hairy roots, transformed with
Agrobacterium rhizogenes, have also been
found to be suitable for the production of secondary metabolites due to their
stability and high productivity in hormone-free culture conditions (Oksman-
Caldentey, 2002; Shanks and Morgan, 1999), and several medicinal plant
species have been successfully transformed in this manner. The selection of high-productivity root lines based on somaclonal variation also offers an interesting
option for enhancing productivity. While this type of metabolite-producing
culture provides stable material and an interesting field of study, how hairy root
formation affects metabolite production is not clear. The hairy root system could
have a more complicated level of regulation due to tissue organization and the
effect of integrated transgenes (see below).
In higher plants and other complex organisms, certain pathways of secondary
metabolism can depend on the general development of the organism, including
organ, tissue, and particular specialized cell development (Wiermann, 1981). The
biosynthesis and accumulation of several secondary compounds occur during
defined developmental stages in an organism. Compounds are not necessarily
synthesized in organs and tissues with high levels of accumulation; for example,
tropane alkaloids in
Atropa and nicotine alkaloids in tobacco are produced in root
and transported to aerial parts (Yun
et al., 1992).
One of the most well-known examples of cell differentiation regarding secondary
metabolites is the glandular trichome for essential oils in mint and related
species (Kutchan, 2005a). These glandular cells show a striking differentiation of
tubular smooth ER, and the biosynthetic characteristics of glandular trichomes
have been examined by EST analysis (Lange
et al., 2000). Highly cytotoxic monoterpenoids
require this specific structure for biosynthesis and accumulation.
While it is not clear whether the process of the formation of glandular trichomes
is similar to that of nonglandular trichomes, the successful trichome formation
seen on all epidermal surfaces by the constitutive overexpression of transcriptional
factor (GL1 and maize R) genes may provide insight into cellular
differentiation and metabolite production (Lange and Croteau, 1999).
The extent to which secondary metabolism depends on the development of
specific cellular structures is not yet clear. Poppy plants have idioblast cells
specialized for the storage of secondary products and laticifers for excretion (Bird
et al., 2003; Facchini and St-Pierre, 2005;Kutchan, 2005a;Weid
et al., 2004).Terpenoid
indole and tropane alkaloids also need similar cellular collaboration (Burlat
et al., 2004; Facchini and St-Pierre, 2005; Kutchan,
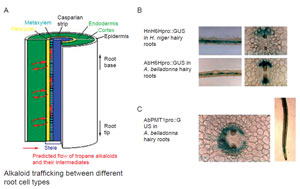 |
| FIGURE 11.6 Cell-specific gene expression in
tropane alkaloids. (A) A model for alkaloid
trafficking between different cell types in root.
(B) Pericycle cell-specific gene expression of the
H6H gene. (C) Cell-specific gene expression of
the PMT gene. |
2005a; St-Pierre
et al., 1999): nonmevalonate
pathway enzymes and G10H to produce 10-hydroxygeraniol are localized in
internal phloemparenchyma cells, TDC and STR, in the early pathway of terpenoid
indole alkaloid biosynthesis, are in epidermal cells, while D4H and DAT, in the late
pathway, are in idioblast cells of aerial organs. In tropane alkaloid biosynthesis, the
entry enzyme,PMT, is expressed in the cortex and endodermis,whereas the last step
of the pathway, H6H, is specifically expressed in pericycle cells in root (Fig. 11.6).
These results indicate that intermediates should drive the site of the primary reaction
to that of the end reaction. The morphological differentiation of cells would be
needed for functional differentiation of each specific reaction in metabolism. However,
the molecular basis of the link between function and morphological differentiation
is not yet clear. Indeed, this observation can explain why the production of
some metabolites requires organ differentiation. For example, the overexpression of
a key enzyme (H6H) in tropane alkaloid biosynthesis can improve the production of
scopolamine in cultured cells (Yun
et al., 1992).





