Freshwater Media
Media are generally prepared from premixed stock solutions. Aliquots from these stocks are measured and added to a given volume of water. Some, however, must be prepared by weighting or measuring the desired components and adding them directly to a given volume of liquid. Accuracy in measuring liquid aliquots from stock solution or water, and weighing of chemicals is essential.
Stock solution can be prepared and stored at low temperature in tightly sealed glassware, because evaporation may alter initial concentrations.
Water generally employed for freshwater media should belong to one of the following types: copper-distilled water; single glass-distilled water; double glass-distilled water; membrane filtered water; and deionized water. In most laboratories single or double-distilled water is routinely used, which can be deionized by passing it through a prepacked deionizing column.
Some of the most commonly used freshwater media, defined and undefined, are listed in:
- Table 6.2: BG11 Medium Composition
- Table 6.3: Diatom Medium Composition
- Table 6.4: DY-III Medium Composition
- Table 6.5: Aaronson Medium Composition
- Table 6.6: Cramer and Myers Medium Composition
- Table 6.7: Beijerinck Medium Composition
- Table 6.8: Bold Basal Medium Composition
- Table 6.9: Mes-Volvox Medium Composition
Seawater is an ideal medium for growth of marine species, but it is an intrinsically complex medium, containing over 50 known elements in addition to a large but variable number of organic compounds. Usually it is necessary to enrich seawater with nutrients such as nitrogen, phosphorus, and iron. Synthetic formulations have been designed primarily to provide simplified, defined media. Marine species generally have fairly wide tolerances, and difficulties attributed to media can frequently be related to problems of isolation, conditions of manipulations and incubation, and physiological state of the organism. A single medium will generally serve most needs of an investigator. Many media are only major variations of some widely applicable, and often equally effective media. Whatever the choice, a medium should be as simple as possible in composition and preparation.
The salinity of the seawater base should first be checked (30–35‰ for marine phytoplankton), and any necessary adjustments (addition of fresh water/evaporation) made before addition of enrichments.
Seawater, stock solutions of enrichments, and the final media must be sterile in order to prevent (or more realistically minimize) biological contamination of unialgal cultures. Autoclaving is a process which has many effects on seawater and its constituents, potentially altering or destroying inhibitory organic compounds, as well as beneficial organic molecules. Because of the steam atmosphere in an autoclave,CO2 is driven out of the seawater and the pHis raised to about 10, a level which can cause precipitation of the iron and phosphate added in the medium. Some of this precipitate may disappear upon re-equilibriation of CO2 on cooling, but both the reduced iron and phosphate levels and the direct physical effect of the precipitate may limit algal growth. The presence of ethylenediaminetetraacetic acid (EDTA) and the use of organic phosphate may reduce precipitation effects. Addition of 5% or more of distilled water may also help to reduce precipitation (but may affect final salinity). The best solution, however, if media are autoclaved, is to sterilize iron and phosphate (or even all media additions) separately and add them septically afterwards.
Seawater Base
The quality of water used in media preparation is very important. Natural seawater can be collected near shore, but its salinity and quality is often quite variable and unpredictable, particularly in temperate and polar regions (due to anthropogenic pollution, toxic metabolites released by algal blooms in coastal waters). The quality of coastal water may be improved by ageing for a few months at 4°C (allowing bacteria degradation), by autoclaving (heat may denature inhibitory substances), or by filtering through acid-washed charcoal (which absorbs toxic organic compounds). Most coastal waters contain significant quantities of inorganic and organic particulate matter, and therefore must be filtered before use (e.g., Whatman no. 1 filter paper).
Artificial seawater, made by mixing various salts with deionized water, has the advantage of being entirely defined from the chemical point of view, but it is very laborious to prepare, and often does not support satisfactory algal growth. Trace contaminants in the salts used are at rather high concentrations in artificial seawater because so much salt must be added to achieve the salinity of full strength seawater. Commercial preparations are available, which consists of synthetic mixes of the major salts present in natural sea water, such as Tropic Marine Sea Salts produced in Germany for Quality Marine (USA) and Instant Ocean Sea Salts by Aquarium System (USA).
Nutrients, Trace Metals, and Chelators
The term ‘nutrient’ is colloquially applied to a chemical required in relatively large quantities, but can be used for any element or compound necessary for algal growth.
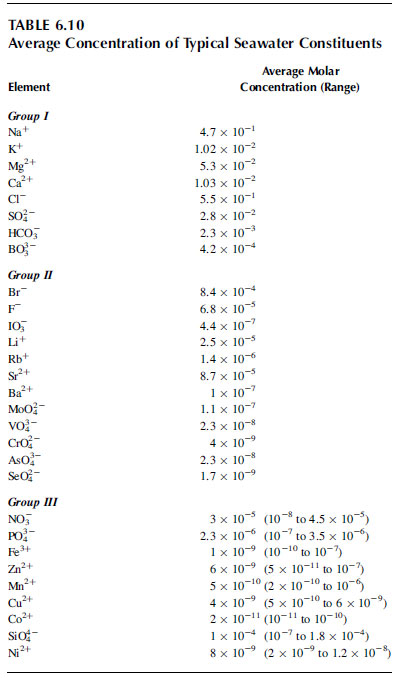
The average concentrations of constituents of potential biological importance found in typical seawater are summarized in Table 6.10. These nutrients can be divided into three groups, I, II, and III; with decreasing concentration:
- Group I. Concentrations of these constituents exhibit essentially no variation in seawater, and high algal biomass cannot deplete them in culture media. These constituents do not, therefore, have to be added to culture media using natural seawater, but do need to be added to deionized water when making artificial seawater media.
- Group II. Also have quite constant concentrations in seawater, or vary by a factor less than 5. Because microalgal biomass cannot deplete their concentrations significantly, they also do not need to be added to natural seawater media. Standard artificial media (and some natural seawater media) add molybdenum (as molybdate), an essential nutrient for algae, selenium (as selenite), which has been demonstrated to be needed by some algae, as well as strontium, bromide, and flouride, all of which occur at relatively high concentrations in seawater, but none of which have been shown to be essential for microalgal growth.
- Group III. All known to be needed by microalgae (silicon is needed only by diatoms and some chrysophytes, and nickel is only known to be needed to form urease when algae are using urea as a nitrogen source). These nutrients are generally present at low concentrations in natural seawater, and because microalgae take up substantial amounts, concentrations vary widely (generally by a factor of 10 to 1000). All of these nutrients (except silicon and nickel in some circumstances) generally need to be added to culture media in order to generate significant microalgal biomass.
Inorganic (ortho)phosphate, the phosphorus form preferentially used by microalgae, is most often added to culture media, but organic (glycero)phosphate is sometimes used, particularly when precipitation of phosphate is anticipated (when nutrients are autoclaved in the culture media rather than separately, e.g.). Most microalgae are capable of producing cell surface phosphatases, which allow them to utilize this and other forms of organic phosphate as a source of phosphorus.
Vitamins
Roughly all microalgal species tested have been shown to have a requirement for vitamin B12, which appears to be important in transferring methyl groups and methylating toxic elements such as arsenic, mercury, tin, thallium, platinum, gold, and tellurium, around 20% need thiamine, and less than 5% need biotin.
It is recommended that these vitamins are routinely added to seawater media. No other vitamins have ever been demonstrated to be required by any photosynthetic microalgae.
Soil extract has historically been an important component of culture media. It is prepared by heating, boiling, or autoclaving a 5–30% slurry of soil in fresh water or seawater and subsequently filtering out the soil. The solution provides macronutrients, micronutrients, vitamins, and trace metal chelators in undefined quantities, each batch being different, and hence having unpredictable effects on microalgae. With increasing understanding of the importance of various constituents of culture media, soil extract is less frequently used.