Control of Microorganisms by using Disinfectants and Antiseptics
There are a number of factors which influence the antimicrobial action of disinfectants and antiseptics, including:
- The concentration of the chemical agent.
- The temperature at which the agent is being used. Generally, the lower the temperature, the lower the effectiveness.
- The kinds of microorganisms present (endospore producers, Mycobacterium tuberculosis, etc.).
- The number of microorganisms present. The more organisms present, the harder it is to disinfect.
- The nature of the material bearing the microorganisms. Organic material such as dirt and excreta interferes with some agents.
The best results are generally obtained when the initial microbial numbers are low and when the surface to be disinfected is clean and free of possible interfering substances.
There are 2 common antimicrobial modes of action for disinfectants and antiseptics:
- They may damage the lipids and/or proteins of the semipermeable cytoplasmic membrane of microorganisms, resulting in leakage of cellular materials needed to sustain life.
- They may denature microbial enzymes and other proteins, usually by disrupting the hydrogen and disulfide bonds that give the protein its 3- dimensional functional shape. This blocks metabolism.
A large number of such chemical agents are in common use. Some of the more common groups are listed below:
- Phenol and phenol derivatives: Phenol (5%–10%) was the first disinfectant commonly used. However, because of its toxicity and odor, phenol derivatives are now generally used. These include orthophenylphenol, hexachlorophene, triclosan, hexylresorcinol, and chlorhexidine. Orthophenylphenol is the agent in Lysol®, O-syl®, Staphene®, and Amphyl®. Hexachlorophene in a 3% solution is combined with detergent and found in PhisoHex®. Triclosan is a phenolic antiseptic very common in antimicrobial soaps and other products. Hexylresorcinol is in throat lozenges and ST-37. A 4% solution of chlorhexidine in isopropyl alcohol and combined with detergent (Hibiclens®) is a common handwashing agent and surgical handscrub. These agents kill most bacteria, most fungi, and some viruses, but are usually ineffective against endospores. They alter membrane permeability and denature proteins.
- Soaps and detergents: Soaps are only mildly microbicidal. Their use aids in the mechanical removal of microorganisms by breaking up the oily film on the skin (emulsification) and reducing the surface tension of water so it spreads and penetrates more readily. Many cosmetic soaps also contain added disinfectants to increase antimicrobial activity. Detergents may be anionic or cationic. Anionic (negatively charged) detergents, such as laundry powders, mechanically remove microorganisms and other materials but are not very microbicidal. Cationic (positively charged) detergents alter membrane permeability and denature proteins. They are effective against many vegetative bacteria, some fungi, and some viruses. However, endospores, Mycobacterium tuberculosis, and Pseudomonas species are usually resistant. They are also inactivated by soaps and organic materials like excreta. Cationic detergents include the quaternary ammonium compounds (zephiran, diaprene, roccal, ceepryn, and phemerol).
- Alcohols: 70% solutions of ethyl or isopropyl alcohol are effective in killing vegetative bacteria, enveloped viruses, and fungi. However, they are usually ineffective against endospores and nonenveloped viruses. Once they evaporate, their cidal activity will cease. Alcohols denature membranes and are often combined with other disinfectants, such as iodine, mercurials, and cationic detergents for increased effectiveness.
- Acids and alkalies: Acids and alkalies alter membrane permeability and denature proteins and other molecules. Salts of organic acids, such as calcium propionate, potassium sorbate, and methylparaben, are commonly used as food preservatives. Undecylenic acid (Desenex®) is used for dermatophyte infections of the skin. An example of an alkali is lye (sodium hydroxide).
- Heavy metals: Heavy metals, such as mercury, silver, and copper, denature proteins. Mercury compounds (mercurochrome, metaphen, merthiolate) are only bacteriostatic and are not effective against endospores. Silver nitrate (1%) is sometimes put in the eyes of newborns to prevent gonococcal ophthalmia. Copper sulfate is used to combat fungal diseases of plants and is also a common algicide. Selinium sulfide kills fungi and their spores.
- Chlorine: Chlorine gas reacts with water to form hypochlorite ions, which in turn denature microbial enzymes. Chlorine is used in the chlorination of drinking water, swimming pools, and sewage. Sodium hypochlorite is the active agent in household bleach. Calcium hypochlorite, sodium hypochlorite, and chloramines (chlorine plus ammonia) are used to sanitize glassware, eating utensils, dairy and food processing equipment, and hemodialysis systems.
- Iodine and iodophores: Iodine also denatures microbial proteins and is usually dissolved in an alcohol solution to produce a tincture. Iodophores are a combination of iodine and an anionic detergent (such as polyvinylpyrrolidone), which reduces surface tension and slowly releases the iodine. Iodophores are less irritating than iodine and do not stain. They are generally effective against vegetative bacteria, Mycobacterium tuberculosis, fungi, some viruses, and some endospores. Examples include Wescodyne®, Ioprep®, Ioclide®, Betadine®, and Isodine®.
- Aldehydes: Aldehydes, such as formaldehyde and glutaraldehyde, denature microbial proteins. Formalin (37% aqueous solution of formaldehyde gas) is extremely active and kills most forms of microbial life. It is used in embalming, preserving biological specimens, and preparing vaccines. Alkaline glutaraldehyde (Cidex®), acid glutaraldehyde (Sonacide®), and glutaraldehyde phenate solutions (Sporocidin®) kill vegetative bacteria in 10 to 30 minutes and endospores in about 4 hours. A 10-hour exposure to a 2% glutaraldehyde solution can be used for cold sterilization of materials.
- Ethylene oxide gas: Ethylene oxide is one of the very few chemicals that can be relied upon for sterilization (after 4–12 hours of exposure). Since it is explosive, it is usually mixed with inert gases such as freon or carbon dioxide. Gaseous chemosterilizers, using ethylene oxide, are commonly used to sterilize heat-sensitive items such as plastic syringes, petri plates, textiles, sutures, artificial heart valves, heart-lung machines, and mattresses. Ethylene oxide has very high penetrating power and denatures microbial proteins. Vapors are toxic to the skin, eyes, and mucus membranes, and are also carcinogenic.
Evaluation of Disinfectants and Antiseptics
It is possible to evaluate disinfectants and antiseptics using either in vitro or in vivo tests. An in vitro test is one done under artificial, controlled laboratory conditions. An in vivo test is one done under the actual conditions of normal use.
A common in vitro test is to compare the antimicrobial activity of the disinfectant being tested with that of phenol. The resulting value is called a phenol coefficient and has some value in comparing the strength of disinfectants under standard conditions. Phenol coefficients may be misleading, however, because as mentioned earlier, the killing rate varies greatly with the conditions under which the chemical agents are used. The concentration of the agent, the temperature at which it is being used, the length of exposure to the agent, the number and kinds of microorganisms present, and the nature of the material bearing the microorganisms all influence the antimicrobial activity of a disinfectant. If a disinfectant is being evaluated for possible use in a given in vivo situation, it must be evaluated under the same conditions in which it will actually be used. We will do a test to see how thermometers might carry microorganisms if not properly disinfected or cleaned.
Effectiveness of Hand Washing
There are 2 categories of microorganisms, or flora, normally found on the hands. Resident flora are the normal flora of the skin. Transient flora are the microorganisms you pick up from what you have been handling. It is routine practice to wash the hands prior to and after examining a patient and to do a complete regimented surgical scrub prior to going into the operating room.
This is done in order to remove the potentially harmful transient flora, reduce the number of resident flora, and disinfect the skin. Actual sterilization of the hands is not possible since microorganisms live not only on the surface of the skin but also in deeper skin layers, in ducts of sweat glands, and around hair follicles. These normal flora are mainly nonpathogenic staphylococci and diphtheroid bacilli. We will qualitatively evaluate the effectiveness of the length of washing time on the removal of microorganisms from the hands.
Procedures
Evaluations of Disinfectants and Antiseptics
Materials
- 8 sterile glass rods (thermometers)
- 12 plates of trypticase soy agar (TSA); 3 each
- 16 tubes of sterile water; 4 each
- 4 tubes of a particular disinfectant; 1 each
- 1 bottle of dishwashing detergent per class
Organisms
- Trypticase soy broth culture of Escherichia coli
- Trypticase soy broth culture of Bacillus subtilis
- Oral sample (your mouth)
- Stool specimen
Disinfectants
- 4 tubes of one of the following disinfectants per group of 4:
- 70% isopropyl alcohol
- 3% hydrogen peroxide
- Brand “X” mouthwash
- Brand “Y” mouthwash
Procedure for Evaluation of Disinfectants and Antiseptics
Each group of 4 will test one particular disinfectant against each of the 4 organisms or samples. One person will test the normal flora of his or her own mouth, one will test a stool specimen, one will test E. coli., and one will test B. subtilis.
- Take 2 plates of TSA and, using your wax marker, divide each plate in half. Label the 4 halves as follows: control, 5 seconds, 30 seconds, and 3 minutes. Also write the name of the disinfectant your group is testing, the name of the specimen being tested, and your group name or symbol on each plate. Take the third TSA plate and label it “soap and water.”
- Holding the sterile glass rod by one tip only, place it in your mouth, in the stool specimen, in the E. coli., or in the B. subtilis for 3 minutes.
- After 2 minutes, place the rod in your first tube of sterile water to rinse it briefly.
- Remove the rod from the water, let the excess water drip off, and streak the tip of the rod on the control sector of the TSA plate. Be careful that the inoculum does not enter the other sector of the plate.
- Place the rod in your mouth (use a new sterile glass rod for the mouth), the stool, the E. coli., or the B. subtilis a second time for 3 minutes. Then place it in your tube of disinfectant for 5 seconds. Remove the rod from the disinfectant and rinse it briefly in your second tube of sterile water. Streak the tip of the rod on the 5-second sector of the TSA plate.
- Place the rod in the specimen a third time (use a new sterile rod for your mouth) for 3 minutes. Then place it in your disinfectant tube for 30 seconds. Rinse it briefly in your third tube of sterile water, and streak the tip on the 30-second sector of the TSA plate.
- Place the rod in the specimen a fourth time (use a new sterile rod for your mouth) for 3 minutes. Then place it in your tube of disinfectant for 3 minutes. Rinse it briefly in your fourth tube of sterile water, and streak the tip on the 3-minute sector of the TSA plate.
- Place the rod in the specimen a final time (use a new sterile rod for your mouth) for 3 minutes. Squeeze a small amount of dishwashing detergent on the rod and clean the rod using a wet paper towel. Rinse the rod under running water and streak the tip of the rod on the TSA plate labeled “soap and water.”
- Incubate the TSA plates at 37°C until the next lab period.
Effectiveness of Hand Washing
Materials
- 2 plates of trypticase soy agar (TSA)
- Sterile scrub brush
- Soap
Procedure for Effectiveness of Hand Washing
- Using your wax marker, divide each TSA plate in half and label the halves 1 through 4.
- Rub your fingers over sector 1 prior to washing your hands.
- Using a scrub brush, soap, and water, scrub your hands for 2 minutes. Rub your damp fingers over sector 2.
- Again scrub your hands with soap and water for 2 minutes and rub your fingers over sector 3.
- Again scrub your hands with soap and water for 2 minutes and rub your fingers over sector 4.
- Incubate the TSA plates at 37°C until the next lab period.
Results
Evaluation of Disinfectants and Antiseptics
Record your group’s results and the results of the other groups who used different disinfectants below:
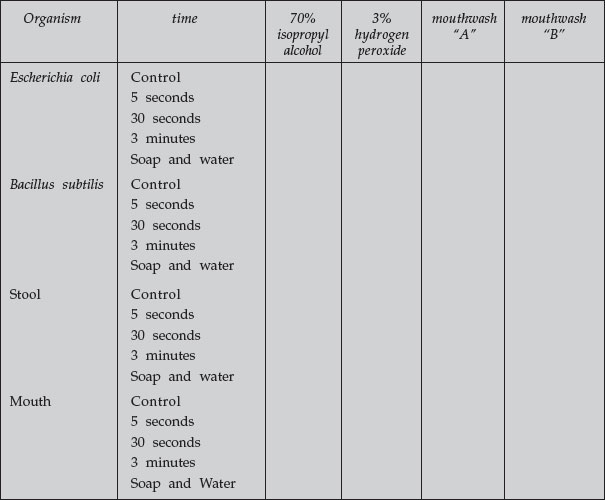 |
– = no growth
+ = some growth
++ = abundant growth
Effectiveness of Hand Washing
Record your results of the 2 TSA “hand washing” plates:
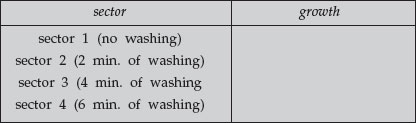 |
– = no growth
+ = some growth
++ = abundant growth
Performance Objectives
Disinfectants and Antisptics
- Define the following terms: disinfection, disinfectant, antiseptic.
- Explain why chemical agents are usually unreliable for sterilization.
- List 5 factors that may influence the antimicrobial action of disinfectants and antiseptics.
- Describe 2 modes of action of disinfectants and antiseptics (i.e., how they harm the microorganisms).
- Name 2 chemical agent that are reliable for sterilization.
Evaluation of Disinfectants and Antiseptics
State why the results of an in vitro test to evaluate chemical agents may not necessarily apply to in vivo situations.
Evaluation of Hand Washing
Define transient flora and resident flora and compare the 2 groups in terms of ease of removal.




