Electrofusion: Nuclear Reprogramming of Somatic Cells by Cell Hybridization with Pluripotential Stem Cells
The technique of cell fusion, which was pioneered by Henry Harris (1965), has proved to be a powerful procedure with applications in cell biology, genetics, and developmental biology and in fields of practical concern such as medicine and agriculture. The spontaneous or induced cell fusion of two different types of cells (heterokaryons) generates intraspecific or interspecific hybrid cells. Genetically programmed spontaneous cell fusion is known to occur in the formation of polykaryons such as myotubes, osteoclasts, and syntrophoblasts in vivo. Under in vitro culture conditions, spontaneous cell fusion has been found to occur occasionally in some cell lines and malignant cells. Cell fusion due to membrane integrity between two different cells is induced by treatment with chemical agents such as calcium ions, lysolecithin, and polyethylene glycol; by mediation by viruses such as paramyxoviruses, including Sendai virus (HVJ), oncornavirus, coronavirus, herpesvirus and poxvirus; and by electrofusion.
This article describes a practical procedure for electrofusion to produce hybrid cells between pluripotential stem cells and committed somatic cells (mouse ES cells and lymphocytes isolated from the adult thymus) without the use of virus or chemicals to mediate the fusion. ES cells are adherent cells that undergo selfrenewal by rapid cell division, whereas thymocytes are nondividing and nonadherent cells. In order to select the hybrid cells effectively, either thymocytes carrying the neo transgene or male ES cells deficient for the Xlinked Hprt (hyoxanthine phosphoribosyl transferase) gene are used as the partner cells in the cell fusion. Consequently, only hybrid cell colonies are capable of surviving and growing in culture in the presence of antibiotic G418 or HAT (hypoxanthine, aminopterin, and thymidine).
Cells: Adult mice, ES cells, and neo r feeder cells (see Section II,A)
Instruments: ECM 2001 AC/DC pulse generator (BTX), 1-mm gap microslide chambers (BTX P/N450- 10WG), inverted microscope with 10 and 20× objectives, humidified incubator at 37°C, 5% CO2, 95% air, 60-mm plastic tissue culture dishes, 60- and 100-mm bacterial dishes, 10- and 30-mm well plastic tissue culture plates, 15- and 50-ml conical tubes, 0.2-µm microfilters, 200- and 1000-µl capacity adjustable pipetters with autoclaved tips, forceps, scissors, 2.5-ml syringes, 18-gauge needles
Compounds: Dulbecco's modified Eagle's medium/ nutrient mixture F12 Ham (DMEM/F12) (Sigma D6421), Dulbecco's modified Eagle's medium (DMEM) (Sigma D5796), fetal bovine serum (FBS) (JRH Biosciences 12003-78P), recombinant leukemia inhibitory factor (LIF) (Chemicon ESG1107), 200 mM glutamine (GIBCO 320-5030AG), 2-mercaptoethanol (Sigma M7520), 10,000 IU/ml penicillin and 10mg/ml streptomycin (penicillin-streptomycin 100×) (Sigma P-0781), 100 mM sodium pyruvate (Sigma S8636), 7.5% sodium bicarbonate (Sigma S8761), Ca2+/Mg2+-free phosphate-buffered saline (PBS) (GIBCO 10010-023), 0.25% trypsin/ 1 mM EDTA. 4Na (GIBCO 25200-056), mytomycin C (Sigma M0503), gelatin from porcine skin, type A (Sigma G-1890), D-mannitol (Sigma M-9546), G418 (geneticin) (Sigma G-9516), HAT media supplement 50x (HAT) (Sigma H0262)
A. Mouse ES Cell and Feeder Cell Culture
One of the most important variables for cell fusion experiments is how stably ES cells (2n = 40) and hybrid cells (2n = 80) can be cultured without loss of the pluripotential competence and the full set of chromosomes derived from mouse ES cells and somatic cells through numerous cell divisions. The culture conditions are basically those described previously (Abbondanzo et al., 1993). A crucial point is quality control of the FBS, which is added to make the ES cell culture medium cocktail. FBS certified for ES cell culturing has become available commercially. We strongly recommend the use of a suitable production lot of FBS that can support effective cell growth without inducing differentiation equivalently to the ES cell-certified FBS.
- ES medium: Mix 500ml of DMEM/F12, 75ml of FBS, 5ml of 200mM glutamine, 5ml of penicillinstreptomycin (100×), 5 ml of 100 mM sodium pyruvate, 8ml of 7.5% sodium bicarbonate, 4µl of 10-4M 2-mercaptoethanol, and 0.05 ml of 107U/ml LIF (final 1000U/ml). Store at 4°C.
- PEF medium: Mix 500ml of DMEM, 50ml of FBS, 5ml of 200mM glutamine, 5ml of 10,000IU/ml penicillin, and 10 mg/ml streptomycin. Store at 4°C.
- 0.25% trypsin/1 mM EDTA.4Na: Dispense into aliquots and store at-20°C.
- Ca2+/Mg2+-free phosphate-buffered saline (PBS)
- lOlag/ml mytomycin C: 0.2mg/ml in PBS, dispense into aliquots, and store at-20°C.
- 0.1% gelatin: 0.1% gelatin in distilled water. Sterilize by autoclaving and store at 4°C.
Steps
- Coat 60-mm culture dishes with 0.1% gelatin for at least 30min at room temperature.
- Prepare mouse primary embryonic fibroblasts (PEFs) produced from day 13 embryos of ROSA26 transgenic mice carrying the ubiquitously expressed neo/lacZ gene (Friedrich and Soriano 1991). Treat neor PEFs with 10µg/ml mitomycin C (MMC) and incubate at 37°C in a CO2 incubator for 2h to produce mitotically inactivated feeder cells. Prepare frozen stocks of the MMC-treated PEFs at a concentration of 5 × 106 cells/ml and store in a cryotube in liquid nitrogen. The inactivated neo r PEFs are routinely used as feeder cells (1 × 106 cells/60- mm culture dish and 2.5 × 106 cells/100-mm culture dish) for culture of ES and hybrid cells, and also for selection of hybrid cell colonies with G418. For establishment of PEFs, see Abbondanzo et al. (1993).
- Culture exponentially growing ES cells on the inactivated PEFs with changes of culture medium once or twice a day. Carry out subculturing of the ES cells every 2 days by a 1:4 split. ES cells at early passages are used for experiments. Before cell fusion, it should be verified that the karyotype of the ES cells is normal.
- Prepare gelatin-coated 30-mm culture dishes (6- well-culture plates) containing the inactivated PEFs (4 × 105 cells/well) in 3ml of ES medium 1 day before cell fusion experiments.
B. Pretreatment of ES and Somatic Cells for Cell Fusion
Solution
Fresh nonelectrolyte solution; 0.3 M mannitol buffer: To make 50ml, dissolve 2.74g of mannitol in distilled water. Filter through a 0.2-µm filter. Store at 4°C.
Steps
- Trypsinize ES cells and remove excess trypsin quickly. Add 3ml of ES medium to inactivate the trypsin and dissociate the cells into a single-cell suspension by gentle pipetting. Plate them on a new gelatin-coated 60-mm culture dish. Incubate the ES cells in a CO2 incubator for 30min to separate feeder cells from ES cells.
- Collect unattached ES cells and harvest them by centrifugation at 1500rpm for 5min. Resuspend the cell pellet in 10ml of DMEM, transfer them into a 15-ml conical tube, and place at room temperature.
- Sacrifice a 6- to 8-week-old adult mouse humanely and dissect out the thymus in a clean room if a clean bench is not available. All of the dissection instruments should be sterilized by immersion in 70% ethanol, followed by flaming.
- Wash the tissues with sterilized PBS twice in 60-mm petri dishes and place one lobe of the thymus in the barrel of a sterile 2.5-ml syringe with a sterile 18-gauge needle.
- To dissociate the thymus into a single-cell suspension, gently expel the thymus through the tip of the needle into 2ml of DMEM in a 50-ml conical tube. Draw up and expel the suspension several times. Allow to stand for several minutes at room temperature.
- Transfer the supernatant excluding cell clumps to a 15-ml conical tube and add 10ml of DMEM.
- Spin down the ES cells and thymocytes separately in 15-ml conical tubes at 1500 rpm for 5 min.
- Wash them with 10ml of DMEM and spin down at 1500rpm for 5min and repeat again to remove FBS completely.
- Add 5-10ml of DMEM and adjust the density of ES cells and thymocytes each to 1 × 106 cells/ml.
- Pellet a 1:5 mixture of ES cells and thymocytes (1 ml of the ES cell suspension and 5 ml of the thymocyte suspension made in step 9). Keep the remaining cells for control experiments.
- Spin down and resuspend the cell pellet in 0.3M mannitol buffer at 6 × 106 cells/ml. Usually, 1 ml of the mixture of ES cells and thymocytes is sufficient for the following fusion experiment. Use the cells immediately for electrofusion (Fig. 1).
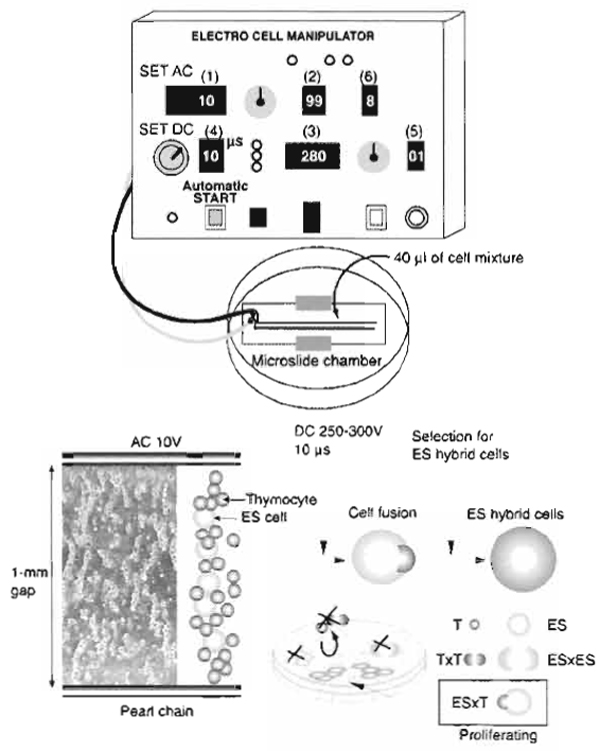 |
| FIGURE 1 Schematic representation of the electrofusion system. A mixture of ES cells and somatic cells suspended in nonelectrolytic mannitol solution is placed in a 1-mm gap between electrodes on a microslide. Set parameters (1-6) of the AC/DC pulse generator and press the automatic start button. AC electric pulses induce pearl chain formation, and the subsequent DC electric pulse induces cell fusion. ES cells (ES) and ES×ES hybrid cells are nonviable in the selection medium, whereas thymocytes (T) and T×T hybrid cells are nonadherent. ES hybrid cells (ES×T) rescued by the thymocyte genome are capable of surviving and proliferating in the selection medium. |
Solution
70% ethanol
Steps
- Sterilize the microslides by immersion in 70% ethanol, followed by flaming.
- Set a microslide in a 100-mm plastic dish chamber.
- For each electrofusion, apply 40 gl of cell mixture between the electrodes with a 1-mm gap on the microslide.
- Connect the microslide in the 100-mm plastic dish chamber with the ECM 2001 AC/DC pulse generator by electric cables. Set the chamber on an inverted microscope to allow for observation of cell alignment and the fusion processes. It is important to monitor the fusion process microscopically each time. The electrofusion may proceed somewhat differently depending on the density of cell preparations and on the cell type (cell size).
- Set the optimized electrical parameters to fuse ES cells and thymocytes (Fig. 1): 10V alternating current (AC), 99s AC duration, and 250-300V direct current (DC). Adjust the DC voltage according to the size of the gap between the electrodes. The appropriate electric field strength is 2.5-3.0 kV/cm. When microslides with a 2-mm gap are used, the DC voltage should be almost 600 V.
DC pulse length: 10 µs
Number of DC pulses: 1
Postfusion AC duration: 8s - Use the automatic operation switch to initiate AC followed by DC. AC is utilized to induce an inhomogeneous, or divergent electric field, resulting in cell alignment and pearl chain formation. DC is utilized to produce reversible temporary pores in the cytoplasmic membranes. When juxtaposed pores in the physically associated cells reseal, cells have a chance to be hybridized via cytoplasmic membrane fusion. AC application after the DC pulse induces compression of the cells, which helps the process of fusion between the cell membranes.
- Add 40µl of DMEM to the fusion mixture between the electrodes to induce the recovery of membrane formation.
- Place the cell mixture at room temperature for 10 min and transfer the cells to a 30-mm culture dish containing inactivated PEFs with 3 ml of the ES medium.
- Repeat the cell fusion procedure sequentially using several microslides. Usually, cells recovered from three microslides (40µl × 3) are plated into one 30-mm culture dish.
- As a control, plate the untreated cell mixture and culture under the same conditions.
- Change the medium to ES medium with appropriate supplements for selecting ES hybrid cells 24h after cell fusion. The selection medium should be changed once a day (see Section III,D). During the 7- day selection treatment, unfused ES cells and hybrid cells between ES cells are killed and hybrid cells between thymocytes are nonadherent. Thus, only the hybrid cells between ES cells and somatic cells survive, proliferate, and form colonies. Several colonies of hybrid cells per 104 host ES cells are obtained under appropriate cell fusion and culture conditions.
- Pick up the colonies with a micropipette and transfer each colony into a 10-mm well of a 24-well culture plate containing 1 × 105 inactivated PEFs per well and 0.8 ml of the ES medium with supplements for selection.
- Subculture the cells every 2 or 3 days and gradually expand the number of cells in 30- and then 60- mm culture dishes with the inactivated PEFs and ES medium with supplements for selection. When the cells become nearly confluent in a 60-mm culture dish, it is considered that a hybrid cell line of passage 1 has been established.
- Change the ES medium without selection supplements once or twice a day and subculture the hybrid cell line every 2 days under optimal culture conditions by splitting 1:4.
- Subject hybrid cells to chromosome analysis soon after they are established.
This section describes one independent chemical selection system that can be used to select for hybrid cells between ES cells and somatic cells.
- Normal ES cells are hybridized with thymocytes containing the bacterial neomycin resistance (neor) gene (Tada et al., 1997, 2001). Thymocytes are derived from ROSA26 transgenic mice, which carry the ubiquitously expressed neo/lacZ transgene (Friedrich and Soriano, 1991). Only ES hybrid cells with the thymocytes can survive and grow in the ES medium supplemented with the antibiotic G418, a protein synthesis inhibitor. In this case, the ES hybrid cells and their derivatives are identified visually by their positive reaction with X-gal due to β-galactosidase activity, allowing one to analyze their contribution to the development of chimeric embryos and tissues (Tada et al., 1997, 2001, 2003). Male ES cells deficient for the Hprt gene on the X chromosome are a powerful tool for producing hybrid cells with wild-type somatic cells. Electrofusion-treated cells are cultured in ES medium with the HAT supplement. In DNA synthesis, purine nucleotides can be synthesized by the de novo pathway and recycled by the salvage pathway. Hprt is a purine salvage enzyme, responsible for converting the purine degradation product hypoxanthine to inosine monophosphate, a precursor of ATP and GTP. In the presence of aminopterin, the de novo pathway is inhibited and only the salvage pathway functions. Consequently, dysfunction of Hprt induces cell death in cultures grown in HAT medium. Thus, the HAT medium proves fatal to Hprt-deficient ES cells, whereas ES hybrid cells, which are rescued by the thymocytes-derived wild-type Hprt gene, are able to survive and proliferate (Tada et al., 2003; Kimura et al., 2003).
Solutions
- ES medium with G418: Reconstitute G418 with water (50mg/ml). Sterilize through a 0.2-µm filter and store at 4°C. Add 50µl of the G418 solution to 10ml of ES medium, yielding a final concentration of 250µg/ml.
- ES medium with HAT: Reconstitute the HAT media supplement obtained from the supplier in a vial with 10 ml of DMEM (50× solution) and store at -20°C. Each vial contains 5 × 10-3 M hypoxanthine, 2 × 10-5 M aminopterin, and 8 × 10-4 M thymidine. Add 200µl of 50× solution to 10ml of ES medium.
Selection with G418
- Perform electrofusion between normal ES cells and thymocytes collected from the 6- to 8-week-old ROSA26 transgenic mice carrying the neo/lacZ transgene according to the procedure described earlier.
- Culture the electrofusion-treated cells in ES medium for 24 h.
- Change to ES medium supplemented with G418. ES hybrid cell colonies can be detected by 7-10 days.
- ES thymocyte hybrid cells are positive for X-gal staining and immunoreactive with the anti-β- galactosidase antibody.
Selection with HAT
- Carry out electrofusion between ES cells (XY) deficient for Hprt and thymocytes collected from 6- to 8-week-old female mice (XX) according to the procedure described earlier.
- Culture electrofusion-treated cells in ES medium for 24h.
- Change to ES medium containing the HAT supplement. ES hybrid cell colonies can be detected by 7-10 days.
- ES thymocyte hybrid cells possess a karyotype of 4n = 80 with an XXXY sex chromosome constitution.
Figure 2A shows representative ES hybrid cells in culture on feeder cells in the ES medium. Figure 2B shows representative neuronal cells differentiated from ES hybrid cells. The ES hybrid cells are pluripotential and can differentiate into a variety of tissues in vivo and in vitro. Tissue-specific transcripts derived from the reprogrammed somatic genomes can be identified based on genetic polymorphisms found in intersubspecific ES hybrid cells (Mus musculus domesticus× M. m. molossinus). The reprogrammed somatic cell genomes function similarly to the ES cell genomes in undifferentiated ES hybrid cells and also in ES hybrid cell derivatives differentiated in vivo and in vitro (Kimura et al., 2003; Tada et al., 2003).
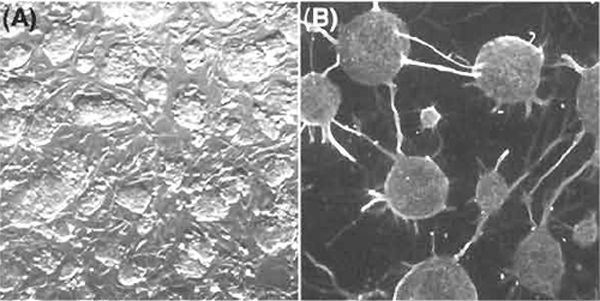 |
| FIGURE 2 Pluripotential competence of ES hybrid cells with somatic cells. (A) Undifferentiated ES somatic hybrid cell colonies in culture on mitotically inactivated PEFs. (B) Neuronal cells differentiated in vitro from ES somatic hybrid cells on PA6 stromal feeder cells. |
ES hybrid cells can also be produced by 50% polyethylene glycol (PEG) treatment. Hybrid cells between embryonic carcinoma (EC) cells deficient for the Hprt gene and lymphocytes from the thymus or spleen are produced by cell fusion induced chemically by PEG (Takagi et al., 1983). To produce ES hybrid cells using PEG, wash a mixture of ES cells and thymocytes with DMEM and pellet the cells by centrifugation. Prewarm 1 ml of a 50% PEG mixture (PEG4000/DMEM = 1:1) at 37°C and then add the PEG mixture to the cell pellet gradually using the tip of a pipette. Add 9ml of DMEM gradually. Collect the cells by centrifugation, resuspend the cells in ES medium, and transfer them to a culture dish. Selection of hybrid cells is begun 1 day after the PEG-induced fusion treatment. Electrofusion has the following advantages over the PEGinduced cell fusion: (1) electrofusion is appropriate for in vivo applications of the hybrid cells, whereas PEGinduced fusion is not because PEG is toxic to cells; (2) electrofusion is more efficient and reproducible than PEG-induced cell fusion for producing ES hybrid cells; and (3) it is easier to produce hybrid cells by electrofusion than by PEG-induced fusion.
V. PITFALLS
If there are problems with the AC procedure, you may be able to solve the problems as follows. Pellet the mixed cells by centrifugation and resuspend the cells in a suitable amount of fresh mannitol buffer.
- Adjust the cell density according to the size of the cells used as the fusion partner. Remove cell debris from the mixture of ES cells and somatic cells. Cell debris, which is irregular in size, sometimes makes the formation of pearl chains difficult.
- Increase the cell density if the pearl chains form poorly. 3. Decrease the cell density if the cell movement is disturbed.Decrease the cell density if the cell movement is disturbed.
Abbondanzo, S. J., Gadi, I., and Stewart, C. L. (1993). Derivation of embryonic stem cell lines. In "Methods in Enzymology; Guide to Techniques in Mouse Development" (E M. Wassarman and M. L. DePamphilis, eds.), pp. 803-823. Academic Press, San Diego.
Baron, M. H., and Maniatis, T. (1986). Rapid reprogramming of globin gene expression in transient heterokaryons. Cell 46, 591-602.
Blau, H. M., and Baltimore, D. (1991). Differentiation requires continuous regulation. J. Cell Biol. 112, 781-783.
Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. E, Silberstein, L., Webster, S. G., Miller, S. C., and Webster, C. (1985). Plasticity of the differentiated state. Science 230, 758-766.
Friedrich, G., and Soriano, E (1991). Promoter traps in embryonic stem cells: A genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513-1523.
Kimura, H., Tada, M., Hatano, S., Yamazaki, M., Nakatsuji, N., and Tada, T. (2003). Chromatin reprogramming of male somatic cellderived Xist and Tsix in ES hybrid cells. Cytogenet. Genome Res. 99.
Tada, M., Morizane, A., Kimura, H., Kawasaki, H., Ainscough, J. E-X., Sasai, Y., Nakatsuji, N., and Tada, T. (2003). Pluripotency of reprogrammed somatic genomes in ES hybrid cells. Dev. Dyn .
Tada, M., Tada, T., Lefebvre, L., Barton, S. C., and Surani, M. A. (1997). Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. EMBO J. 16, 6510-6520.
Tada, M., Takahama, Y., Abe, K., Nakatsuji, N., and Tada, T. (2001). Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 11, 1553-1558.
Tada, T., Tada, M., Hilton, K., Barton, S. C., Sado, T., Takagi, N., and Surani, M. A. (1998). Epigenotype switching of imprintable loci in embryonic germ cells. Dev. Gene Evol. 207, 551-561.
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., Meyer, E. M., Morel, L., Petersen, B. E., and Scott, E. W. (2002). Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542-545.
Vassilopoulos, G., Wang, P. R., and Russell, D. W. (2003). Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901-904.
Wang, X., Willenbring, H., Akkari, Y., Torimaru, Y., Foster, M., A1- Dhalimy, M., Lagasse, E., Finegold, M., Olson, S., and Grompe, M. (2003). Cell fusion is the principal source of bone-marrowderived hepatocytes. Nature 422, 897-901.
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., and Campbell, K. H. S. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810-813.
Ying, Q. L., Nichols, J., Evans, E. P., and Smith, A. G. (2002). Changing potency by spontaneous fusion. Nature 416, 545-548.




