Laboratory Cultivation of Caenorhabditis elegans and Other Free-Living Nematodes
Nematodes have been cultured continuously in the laboratory since 1944 when Margaret Briggs Gochnauer isolated and cultured the free-living hermaphroditic species Caenorhabditis briggsae. Work with C. briggsae and other rhabditid nematodes, C. elegans, Rhabditis anomala, and R. pellio, demonstrated the relative ease with which they could be cultured (Dougherty, 1960; Vanfleteren, 1980). The culturing techniques described here were developed for C. elegans, but are generally suitable (to varying degrees) for other free-living nematodes. Whereas much of the early work involved axenic culturing, most of these techniques are no longer in common use and are not included here.
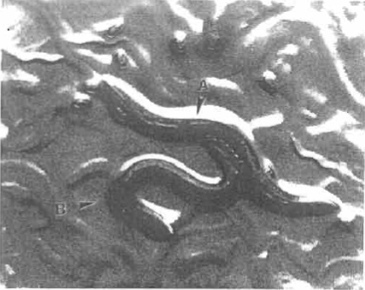 |
| FIGURE 1 Caenorhabditis elegans hermaphrodite (A) and male (B) copulating. Several eggs and young larvae are also visible. |
Many of the protocols included here and other experimental protocols have been summarized in Wood (1988). We also include a previously unpublished method for long-term chemostat cultures of C. elegans. General laboratory culture conditions for nematode parasites of animals have been described (Hansen and Hansen, 1978), but none of these nematodes can be cultured in the laboratory through more than one life cycle. Marine nematodes and some plant parasites have been cultured xenically or with fungi (Nicholas, 1975). Laboratory cultivation of several plant parasites on Arabidopsis thaliana seedlings in agar petri plates has also been reported (Sijmons et al., 1991).
Caenorhabditis elegans strains, as well as strains of other free-living nematodes, and bacterial food sources for them are available from the Caenorhabditis Genetics Center (250 Biological Sciences Center, University of Minnesota, 1445 Gourtner Ave., St. Paul, MN 55108). Most chemicals are obtained from general laboratory supply companies such as Fisher Scientific and Sigma; catalog numbers are for Fisher except where noted. Three sizes of polystyrene petri dishes are used in culturing nematodes: 35 × 10mm (Cat. No. 8-757-100-A), 60 × 15mm (Cat. No. 8-757-13-A), and 100 × 15mm (Cat. No. 8-757-13). Triple-baffled Fernbach flasks (250 ml, 1 liter, and 2.8 liter, Cat. No. 2554) for liquid culture are from Bellco. The programmable dispensing pump (Model DP-200) used when making plates and the chemostat (Bioflo I) are from New Brunswick Scientific Company. An IEC clinical centrifuge (Cat. No. 05-101- 5) with rotor 221 and metal shields 303 at setting 4 (RCF approximately 750) for 30s is used for pelleting nematodes.
III. PROCEDURES
A. Preparation of Plates
The procedure for preparation of plates is modified from that of Brenner (1974).
Solutions
- Cholesterol stock (5 mg/ml): Dissolve 0.5g cholesterol (Cat. No. C314) in a final volume of 100ml of 95% ethanol.
- 1M CaCl2: Dissolve 14.7g of CaCl2.2H2O (Cat. No. C79) in a final volume of 100ml of distilled water and autoclave.
- 1M MgS04: Dissolve 24.65 g of MgSO4.7H2O (Cat. No. M63) in a final volume of 100ml of distilled water and autoclave.
- KH2P04 stock (1M): Dissolve 68.04g of KH2PO4 (Cat. No. P285) in approximately 425ml of distilled water. Add KOH (Cat. No. P250) pellets while monitoring the pH until pH is 6.0. Bring the volume to 500ml; autoclave in 100-ml aliquots.
- B broth: Add 1.0g of tryptone and 0.5g of NaCl (Cat. No. S640) to 100 ml of distilled water in a 250-ml screw-cap flask and autoclave.
- OP50 stock: Inoculate 100 ml of B broth (in screwcap flask) with Escherichia coli strain OP50, a uracil auxotroph, and shake overnight at 37°C. Store the stationary-phase culture at 4°C for up to 60 days.
- NG agar: Add 3 g of NaCl, 17 g of Difco agar (Cat. No. DF0140-01-0), 2.5g of peptone (Cat. No. DF0118- 15-2), and 975ml of distilled water to a 2-liter Erlenmeyer flask. Autoclave. Place the flask in a 50°C water bath to prevent solidification while dispensing medium into plates. Allow the flask to cool to approximately 65°C and add the following, using sterile technique and swirling the flask after adding each ingredient: 1 ml cholesterol stock, 1 ml 1 M CaCl2, 1 ml 1 M MgSO4, and 25 ml KH2PO4 stock.
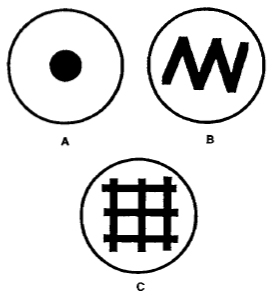 |
| FIGURE 2 Examples of seeded plates. (A) Spot plate used for matings with single males or with mutant animals that do not mate well. (B) Zig-zag plate used for routine strain maintenance and crosses. (C) Grid plate used for mutant screens, strain maintenance, or crosses in which progeny are counted. |
- Accurate dispensing of medium is accomplished most easily with the aid of a programmable dispensing pump. Fill 60 × 15-mm plates with 13ml of agar, 100 × 15-mm plates with 30ml, and 35 × 10-mm plates with 4ml. The plates should be bubble free; flame the surface to remove bubbles.
- Allow the plates to cool overnight; then put plates at 37°C for 24h. Allow the plates to return to room temperature; store at 4°C.
- Seed the plates (Fig. 2) with OP50 stock by spreading approximately 0.05 ml on the surface using a 1-ml pipette, and incubate overnight at 37°C or for 24-48 h at room temperature. Plates should be at room temperature before placing worms on them.
B. Liquid Culture
The procedure is modified from that of Sulston and Brenner (1974).
Solutions
- M9 buffer: Dissolve 3 g of KH2PO4, 6 g of Na2HPO4 (Cat. No. S393), and 5 g of NaCl in distilled water; then add 1 ml of 1M MgSO4. Bring the volume to 1 liter with distilled water and autoclave in 100-ml aliquots.
- S basal: Add 5.84 g of NaCl, 50ml KH2PO4 stock, and 1 ml cholesterol stock to 950ml of distilled water; autoclave in 100-ml aliquots.
- Potassium citrate stock (1M): Add 105.07 g of citric acid monohydrate (Cat. No. A104) to 250ml of distilled water. Add KOH pellets while monitoring the pH until pH is 6.0. Bring the volume to 500ml and autoclave in 100-ml aliquots.
- 100x trace metals: Dissolve 0.69g of FeSO4.H2O (Cat. No. 1467), 1.86g of Na2EDTA (Cat. No. 02793), 0.197 g of MnCl2.4H2O (Sigma, Cat. No. M-3634), 0.287 g of ZnSO4.7H2O (Cat. No. Z76), and 0.025g of CuSO4.5H2O (Cat. No. C493) in 1 liter of distilled water. Autoclave in 100-ml aliquots; store in foilwrapped bottles.
- 50% glucose: Add 50g of glucose (Cat. No. D16) to 50ml of distilled water and autoclave.
- 60% sucrose: Add 120 g of sucrose (Cat. No. S5) to 80ml distilled water and autoclave. Store at 4°C.
- S medium: Add in the order indicated, using sterile technique, 1 ml potassium citrate stock, 1 ml 100x trace metals, 0.3 ml 1M CaCl2, and 0.3 ml 1M MgSO4 to 100 ml of S basal.
- XI666 medium: Add 20g of Na2HPO4·7H2O (Cat. No. S373), 4.5g of KH2PO4, 1.2 g of NH4Cl (Cat. No. A661), 16g of tryptone (Cat. No. DF0123-15-5), and 4g of yeast extract (Cat. No. DF0127-15-1) to 980ml distilled water in a 2.8-liter baffled flask. Mix, autoclave, allow to cool, and add 20ml of 50% glucose and 8 ml of 1M MgSO4.
- XI666 stock: Inoculate X1666 medium with X1666, a nalidixic acid-resistant, prototrophic, plasmid-free strain of E. coli. Shake at 37°C overnight. Transfer to preweighed sterile centrifuge bottles/tubes and centrifuge at 4000 RCF for 10min. Remove supernatant and determine weight of bacteria. Resuspend in S medium to 5% (w/w). Store at 4°C.
Steps
- With 2 ml of M9 buffer per plate, wash worms off five 60 × 15-mm plates that have just cleared of bacteria. Pellet nematodes. Remove supernatant and wash twice with fresh M9.
- Add the washed worms to 250ml X1666 stock in a l-liter sterile baffled flask and place on shaker at 20°C. When the medium is cleared of bacteria, centrifuge at 750 RCF for 5 min.
- Remove supernatant and resuspend in 15 ml of M9 buffer, divide between two 15-ml tubes, and place on ice. When cold, add 7.5 ml of cold 60% sucrose to each tube. Mix by inversion and centrifuge immediately at 1500 RCF for 5 min.
- Remove nematodes from top of tube immediately and wash twice with 15 ml of M9 buffer. Place at 20°C on shaker for 30min to allow digestion of bacteria in nematode intestines.
- Wash twice with M9 buffer and use immediately or freeze at-70°C.
C. Chemostat Culture
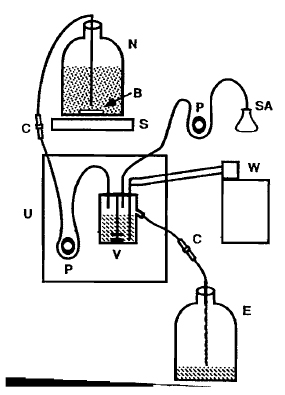 |
| FIGURE 3 Chemostat assembly. N, nutrient reservoir; V, culture vessel; E, effluent tank; S, large stir plate; B, large stir bar (in reservoir to keep bacteria evenly suspended); W, refrigerated circulating water bath (set at 12°C) connected to cold finger of chemostat culture vessel; U, Bioflo I control unit; P, peristaltic pump; SA, selective agent; C, compression fitting (Swagelok Co.) used in the line for easy reservoir replacement (see Section III, C, step 12). Sterility is critical for the maintenance of long-term cultures. |
0P50 concentrate: Make in same manner as X1666 stock except use E. coli strain OP50 and resuspend the pellet in M9 buffer (1/20th volume, or less, of the overnight culture).
Steps
Figure 3 is a diagram of the chemostat assembly.
- Fill nutrient reservoir with 10 liters of 1/5 × S basal [substitute polyoxyethanyl-cholesteryl sebacate (Sigma, Cat. No. C-1145) for cholesterol] and add large stir bar.
- Fill culture vessel with 0.2 vol water. Attach autoclavable 0.2-jiim filters to the vents and air entry tubing. Autoclave connected nutrient reservoir, culture vessel, and effluent tank.
- When cool, add the other stock solutions, as to complete 1/5 × S medium (see Section B), to the nutrient reservoir.
- Add the concentrate from 12 liters of overnight OP50 cultures to the nutrient reservoir (final OD600nm = ~3.7).
- Install culture vessel in chemostat control unit and make connections.
- Start air flow and stir the culture vessel impeller at 220rpm. Set the heat regulator to 20°C.
- Run the feeding pump until culture vessel is full; then turn it off.
- Inoculate culture vessel with sterile LI larvae prepared as described in Section III,E, steps 10 and 11.
- Add OP50 concentrate from a l-liter overnight culture to culture vessel. Add approximately 1 ml sterile antifoam A (Sigma, Cat. No. A-5758) as necessary to minimize foam.
- Monitor the culture every 2 days by removing a sample and counting replicate aliquots spotted on plates (see Section III,A). Continue to add sterile antifoam A to culture vessel as needed. When the culture reaches ~50 animals per 100/A, turn on the feeding pump (setting 9).
- Monitor population density and adjust the speed of the feeding pump as necessary to maintain a reproducing culture. A dense population (~125 animals/10//I) that is mostly LI and L2 larvae and roughly one-fourth dauer larvae can be maintained.
- Prepare a replacement nutrient reservoir for use when the first one is depleted. The effluent tank should be changed at the same time.
D. Freezing Strains for Long-Term Storage
The procedure is modified from that of Brenner (1974).
- 1M NaCI: Dissolve 29.22g of NaCl in a final volume of 500ml distilled water and autoclave.
- S + glycerol: Add 20ml of 1M NaCl, 10ml of KH2PO4 stock, and 60ml of glycerol (Cat. No. G33) to 110 ml distilled water and autoclave.
Steps
- Take three contaminant-free 60 × 15-mm plates 1 day after food is depleted and wash worms off plates with 2ml M9 buffer.
- Add equal volume of S + glycerol and mix by brief vortexing. Transfer in 0.5-ml aliquots to 2-ml cryovials (Vangard International, Cat. No. MS4502). Place vials in a styrofoam freezing box (a styrofoam block with holes the size of cryovials and a styrofoam lid) and immediately put at -70°C (cool at approximately l°C /min).
- After 6h the vials can be transferred to liquid nitrogen or to standard -70°C freezer boxes.
- One vial should be thawed to check for viability, strain accuracy, and microbial contamination. Thaw vial by warming between hands until liquid; then pour contents onto seeded plate. Transfer young healthy worms to fresh plates the next day.
E. Isolation of Staged Animals
Solutions
- Dauer-inducing pheromone stock (modified from Golden and Riddle, 1984): Take 1 liter of starved liquid culture. Reduce volume 75% by evaporation under a stream of air at 100 °C. Centrifuge at 10,000 RCF for 10 min. Dry the supernatant completely at 60 °C. Extract four to six times with 50ml of 95% ethanol until the extract is only slightly colored. Combine the extracts and dry under a stream of air at 60 °C. Back-extract the resulting oily residue with 10ml distilled water. Filter through Whatman 3 MM paper and store at 4 °C.
- 0P50 strep: Transfer OP50 stock (see Section III, A) to preweighed sterile centrifuge bottles/tubes and centrifuge at 4000 RCF for 10 min. Remove supernatant and determine weight of bacteria. Resuspend in S medium to 5% (w/w). Add streptomycin (Sigma, Cat. No. S-6501) to 50/µg/ml final concentration. Store at 4 °C for a maximum of 2 days.
- Wash the worms off approximately five 60 × 15- mm plates containing a large number of gravid adults with approximately 2 ml M9 buffer per plate.
- Combine in a 15-ml Corning polystyrene conical centrifuge tube and pellet nematodes. Remove all but 8 ml of the liquid.
- Mix together 0.5ml 5 N KOH and 1.2ml 20% NaOCl (Cat. No. SS290) in a separate tube; combine with M9 and worms and vortex briefly.
- Remove a small aliquot and monitor under a dissecting scope while agitating the remaining sample gently. When 50 to 75% of the adults in the sample have broken open, pellet nematodes.
- Remove the supernatant, add 8ml of fresh M9 buffer, and pellet. Repeat two more times leaving 0.5- ml volume after the last wash.
- Resuspend the eggs and pipette onto plates. This method will generally leave some carcass parts.
Dauer Larvae
This procedure should give greater than 80% dauers.
- Make NG agar without peptone (see Section III,A). Add approximately 25 ∧1/ml dauer-inducing pheromone stock just before pouring (make only as much as will be used immediately). Pour 2 ml per 35× 10-mm plate.
- After plates solidify, spot with 10µl OP50 strep solution and allow to dry.
- Add approximately 100 eggs or allow adults to lay 100 eggs; then remove the adults and incubate at 25°C for 48-60 h.
Other Stages
- Follow the egg isolation procedure through step 5.
- Bring volume to 10 ml with fresh M9 buffer; incubate on a rocker for 12h or overnight.
- Feed synchronized L1 larvae from the previous step. At 20°C mid-L1 larvae can be harvested after approximately 8h, mid-L2 larvae at 18h, mid-L3 larvae at 25h, and L4 larvae at 37h (Byerly et al., 1976).
For genetic analysis and maintenance of strains in active use, the nematodes should be grown on 60 × 15- mm petri plates. When large numbers of worms are needed, 100 × 15-mm plates are used. When special additives are being used that are expensive or in limited supply, 35 × 10-mm plates are used. Long-term storage of all strains and those not in active use is best accomplished by freezing in liquid nitrogen. For biochemical purposes the nematodes should be grown in liquid culture. Chemostat culturing enables selection on a continuously reproducing population whose density is held constant (Dykhuizen and Haiti, 1983). Most commonly, selection is for an altered growth rate, whether due to induced or spontaneous mutations. Liquid or gaseous selective agents are compatible with the system described.
More precise synchronization for L4 larvae and adults can be accomplished by synchronizing through the dauer stage. If large numbers of dauers are needed, they can be obtained by slightly modifying the liquid culturing procedure. The culture should be allowed to continue for 2-3 days after clearing. After sucrose flotation, treat with a sterile solution of 1% sodium dodecyl sulfate (SDS) for 1 hr [resuspend in 22.5 ml of M9 and add 2.5ml of 10% SDS (dissolve 10g of SDS, Cat. No. S529, in a final volume of 100ml distilled water)]. Wash twice with M9 buffer and then repeat liquid culture protocol starting with step 3.
References
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94.
Byerly, L., Cassada, R. C., and Russell, R. L. (1976). The life cycle of the nematode Caenorhabditis elegans. I. Wild type growth and reproduction. Dev. Biol. 51, 23-33.
Dykhuizen, D. E., and Haiti, D. L. (1983). Selection in chemostats. Microbiol. Rev. 47(2), 150-168.
Golden, J. W., and Riddle, D. L. (1984). The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev. Biol. 102, 368-378.
Hansen, E. L., and Hansen, J. W. (1978). In vitro cultivation of nematodes parasitic on animals and plants. In "Methods of Cultivating Parasites in Vitro" (A. E. R. Taylor and J. R. Baker, eds.), pp. 227-278. Academic Press, London.
Sijmons, P. C., Grundler, E M. W., von Mende, N., Burrows, P. R., and Wyss, U. (1991). Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J. 1, 245-254.
Sulston, J. E., and Brenner, S. (1974). The DNA of Caenorhabditis elegans. Genetics 77, 95-104.
Vanfleteren, J. R. (1980). Nematodes as nutritional models. In "Nematodes as Biological Models" (B. M. Zuckerman, ed.). Vol. 2, pp. 47-79. Academic Press, New York.
Wood, W. B. (1988). "The Nematode Caenorhabditis elegans" (W. B. Wood, ed.), pp. 1-16. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.




