How Cells Are Studied
How Cells Are Studied
Light microscopes, with all their variations
and modifications, have contributed
more to biological investigation
than any other instrument
developed by humans. They have been
powerful exploratory tools for 300
years, and they continue to be so more
than 50 years after invention of the
electron microscope. However, electron
microscopy has vastly enhanced
our appreciation of the delicate internal
organization of cells, and modern biochemical,
immunological, physical, and
molecular techniques have contributed
enormously to our understanding of
cell structure and function.
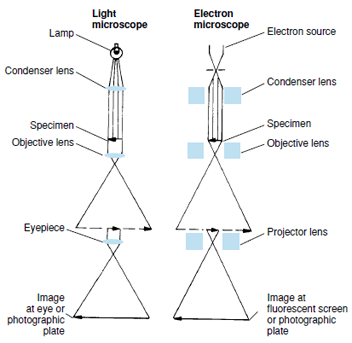
Electron microscopes employ high voltages to direct a beam of electrons through objects examined. The wavelength of the electron beam is approximately 0.00001 that of ordinary white light, thus permitting far greater magnification and resolution (compare A and B of Figure 3-1). In preparation for viewing, specimens are cut into extremely thin sections (10 nm to 100 nm thick) and treated with “electron stains” (ions of elements such as osmium, lead, and uranium) to increase contrast between different structures. Images are seen on a fluorescent screen and photographed (Figure 3-2). Because electrons pass through the specimen to the photographic plate, the instrument is called a transmission electron microscope.
In contrast, specimens prepared for scanning electron microscopy are not sectioned, and electrons do not pass through them. The whole specimen is bombarded with electrons, causing secondary electrons to be emitted. An apparent three-dimensional image is recorded in the photograph. Although the magnification capability of scanning instruments is not as great as transmission microscopes, much has been learned about the surface features of organisms and cells.
A still greater level of resolution can be achieved with X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy. These techniques reveal a great deal about the shape of biomolecules and the relationship of the atoms within them to each other. Both techniques are laborious, but NMR spectroscopy does not require purification and crystallization of a substance, and molecules can be observed in solution.
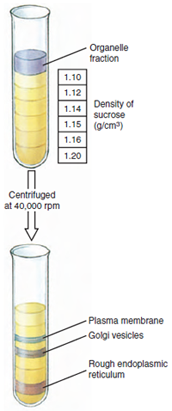
Advances in techniques of cell study (cytology) are not limited to improvements in microscopes but include new methods of tissue preparation, staining for microscopic study, and the great contributions of modern biochemistry and molecular biology. For example, the various organelles of cells have differing, characteristic densities. Cells can be broken up with most of the organelles remaining intact, then centrifuged in a density gradient (Figure 3-3), and relatively pure preparations of each organelle may be recovered. Thus the biochemical functions of various organelles may be studied separately. DNA and various types of RNA can be extracted and studied. Many enzymes can be purified and their characteristics determined. The use of radioactive isotopes has allowed elucidation of many metabolic reactions and pathways in cells. Modern chromatographic techniques can separate chemically similar intermediates and products. A particular protein in cells can be extracted and purified, and specific antibodies against the protein can be prepared. When the antibody is complexed with a fluorescent substance and the complex is used to stain cells, the complex binds to the protein of interest, and its precise location in cells can be determined. Many more examples could be cited, and these have contributed enormously to our present understanding of cell structure and function.
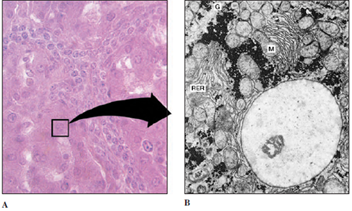 |
| Figure 3-1 Liver cells. A, Magnified approximately 400 times through light microscopy. Note the prominently stained nucleus in each polyhedral cell. B, Portion of single liver cell, magnified approximately 5000 times by electron microscopy. A single large nucleus dominates the field; mitochondria (M), rough endoplasmic reticulum (RER), and glycogen granules (G), are also seen. |
Electron microscopes employ high voltages to direct a beam of electrons through objects examined. The wavelength of the electron beam is approximately 0.00001 that of ordinary white light, thus permitting far greater magnification and resolution (compare A and B of Figure 3-1). In preparation for viewing, specimens are cut into extremely thin sections (10 nm to 100 nm thick) and treated with “electron stains” (ions of elements such as osmium, lead, and uranium) to increase contrast between different structures. Images are seen on a fluorescent screen and photographed (Figure 3-2). Because electrons pass through the specimen to the photographic plate, the instrument is called a transmission electron microscope.
In contrast, specimens prepared for scanning electron microscopy are not sectioned, and electrons do not pass through them. The whole specimen is bombarded with electrons, causing secondary electrons to be emitted. An apparent three-dimensional image is recorded in the photograph. Although the magnification capability of scanning instruments is not as great as transmission microscopes, much has been learned about the surface features of organisms and cells.
A still greater level of resolution can be achieved with X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy. These techniques reveal a great deal about the shape of biomolecules and the relationship of the atoms within them to each other. Both techniques are laborious, but NMR spectroscopy does not require purification and crystallization of a substance, and molecules can be observed in solution.
Advances in techniques of cell study (cytology) are not limited to improvements in microscopes but include new methods of tissue preparation, staining for microscopic study, and the great contributions of modern biochemistry and molecular biology. For example, the various organelles of cells have differing, characteristic densities. Cells can be broken up with most of the organelles remaining intact, then centrifuged in a density gradient (Figure 3-3), and relatively pure preparations of each organelle may be recovered. Thus the biochemical functions of various organelles may be studied separately. DNA and various types of RNA can be extracted and studied. Many enzymes can be purified and their characteristics determined. The use of radioactive isotopes has allowed elucidation of many metabolic reactions and pathways in cells. Modern chromatographic techniques can separate chemically similar intermediates and products. A particular protein in cells can be extracted and purified, and specific antibodies against the protein can be prepared. When the antibody is complexed with a fluorescent substance and the complex is used to stain cells, the complex binds to the protein of interest, and its precise location in cells can be determined. Many more examples could be cited, and these have contributed enormously to our present understanding of cell structure and function.
|
|