Labelling of probes
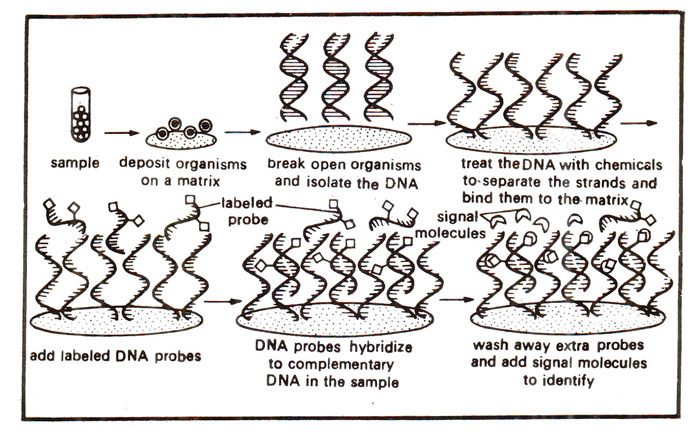
Fig 39.16 Use of labelled DNA probe for identification of homologous DNA sequence through DNA hybridization.
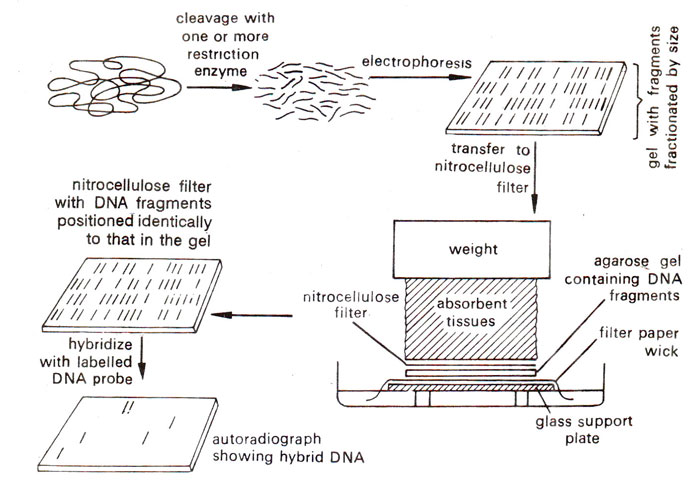
Fig. 39. 3. Southern blotting method of analysing DNA segments that share homology with a nucleic acid probe.
After hybridization with radioactively labelled probe, hybrids are detected by autoradiography (Fig. 39. 3). 32P has the advantage over other radioisotopes, since it has high specific activity. However, in general, radioisotopes have some disadvantages. They are difficult to handle and expensive to dispose off. Detection by autoradiography, while sensitive, may take a long time if there are few counts in the hybrid. Furthermore, radioisotopes have a short half life (e.g. 32P has a half-life of 14.3 days) and therefore experiments should be completed preferably within one half-life.

Fig 39.16 Use of labelled DNA probe for identification of homologous DNA sequence through DNA hybridization.

Fig. 39. 3. Southern blotting method of analysing DNA segments that share homology with a nucleic acid probe.
Digoxigenin is another chemical derived from plants and used for non- radioactive labelling of probes. An antibody associated with an enzyme (anti- digoxigenin-alkaline phosphatase conjugate) is used for the detection of the presence of digoxigenin. The probe may be labelled with digoxigenin-11-dUTP supplied with a digoxigenin kit (these kits are available from any commercial firm, e.g. Boehringer Mannheim). The labelled and denatured probe may be used for hybridization with denatured DNA on Southern blots or for in-situ hybridization. After hybridization the membrane or the slide may be washed and the membrane or the slide is transferred into detection buffer containing 20 μg/ml of anti-digoxigenin fluorescein and 5% (w/v) BSA (bovine serum albumin). The system is incubated for 1h at 37°C, and then the membrane/slide is washed in detection buffer three times (8 min each at 37°C). Alkaline phosphatase activity can be detected using 017 mg/ml BCIP (5-bromo 4-chloro 3-indolyl phosphate) and 0-33 mg/ml NBL (nitroblue tetrazolium) as dye substrate




