Cell-Specific Expression of Monoterpenoid Indole Alkaloid Biosynthetic Genes
The differentiation of plant cells into specialized structures can result in a biochemical
specialization essential for the biosynthesis and accumulation of selected
classes of alkaloids. The lack of cytodifferentiation is considered a possible reason
for the failure of plant cell cultures to accumulate alkaloids such as the dimeric
monoterpenoid indole alkaloid vinblastine. This chemotherapeutic alkaloid is
formed by the oxi
dative coupling of vindoline and catharanthine.
C. roseus cell
cultures accumulate catharanthine, but not vindoline. At least three enzymes of
vindoline biosynthesis are absent from cell culture (reviewed in Kutchan, 1998).
This could be due to the absence of the correct differentiated cell types in culture.
A detailed study of the expression of several genes of vindoline biosynthesis in
developing
C. roseus leaves using
in situ hybridization and immunolocalization
provides the first clear insight into the spatial distribution of monoterpenoid
indole alkaloid biosynthesis (St-Pierre
et al., 1999).
in situ hybridization of two
genes,
tdc and
str1, occurring early in the vindoline biosynthetic pathway and
two genes,
d4h and
dat, occurring late in the vindoline biosynthesis revealed that
multiple cell types are involved.
C. roseus produces more than 180 monoterpenoid indole alkaloids all of which are derived from the central intermediate 3α(S)-
strictosidine. The enzymes tryptophan decarboxylase and strictosidine synthase
lead to this central intermediate and are, therefore, involved in the biosynthesis of
all of the
C. roseus alkaloids. The transcripts of
tdc and
str1 were found in the
epidermis of developing leaves (Fig. 10.4A–D, K, and L) (Irmler
et al., 2000;
St-Pierre
et al., 1999). In contrast, transcripts of the vindoline-specific biosynthetic
genes
d4h and
dat localized to laticifer and idioblasts of developing leaves
(Fig. 10.4E–H, M, and N) (Irmler
et al., 2000; St-Pierre
et al., 1999). In addition,
transcript of the secologanin biosynthetic enzyme
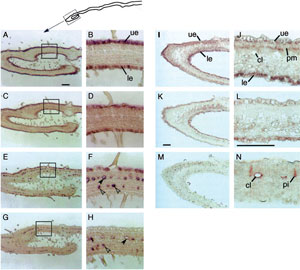 |
| FIGURE 10.4 Localization of cyp72a1, tdc, str1,
d4h, and dat transcripts in developing C. roseus
leaves (Irmler et al., 2000; St-Pierre et al., 1999).
Panels (A, B, K, L) tdc; panels (C, D) str1;
panels (E, F) d4h; panels (G, H, M, N) dat;
panels (I, J) cyp72a1. cl, cross-connecting
laticifer cells; le, lower
epidermis; pi, idioblast
cells associated with palisade mesophyll cells; pm,
palisade mesophyll cells;
si, idioblast cells
associated with spongy mesophyll cells; ue, upper
epidermis; tdc, tryptophan
decarboxylase; str1,
strictosidine synthase; cyp72a1, secologanin
synthase; d4h, desacetoxyvindoline
4-hydroxlyase; dat, deacetylvindoline
4-O-acetyltransferase. Bar shown in panels
(A, K),
100 µm; in panel (L), 50 µm. Solid
arrows in panels (F, H), laticifer cells; open
arrows, idioblast cells. |
secologanin synthase (
cyp72a1)
localized to epidermis of developing leaves is consistent with this tissue as
the site of formation of the central intermediate 3α(S)-strictosidine (Fig. 10.4I
and J; Irmler
et al., 2000). Immunolocalization studies corroborated these results (Irmler
et al., 2000; St-Pierre
et al., 1999). Taken together, interpretation of these
results implies a translocation of a pathway intermediate. They also suggest that a
central alkaloid pathway may occur in one cell type and branches in the pathway
take place in various other cell types. Cellular localization and intermediate
transport can therefore be one level of regulation of alkaloid biosynthesis.
Transcript of
tdc and
str1 are also found in protoderm and cortical cells around
the apical meristem of root tips (St-Pierre
et al., 1999). Likewise, the transcript of a
gene involved in the biosynthesis of the root-specific monoterpenoid indole
alkaloid minovincinine was detected in root tissue. RNA
in situ hybridization
studies located minovincinine 19-hydroxy-O-acetyltransferase gene expression
within the cortex and epidermis of tissues near the root tip (Laflamme
et al., 2001).
Multicellular compartmentation of alkaloid biosynthesis should be a central
consideration in the metabolic engineering of alkaloid pathways. Promoters
should be chosen that would direct transgene expression to the cell types in which
the appropriate biosynthetic intermediates are expected to occur or in which
biosynthetic genes are expressed.





