Cell-Specific Expression of Tetrahydrobenzylisoquinoline Alkaloid Biosynthetic Genes
The opium poppy contains specialized internal secretory cells called laticifers.
In the aerial parts of the plant, the laticifer cells are anastomosed, forming a
reticulated network. Laticifers are found associated with the vascular bundle in
all plant parts. Morphine is found both in roots and in aerial plant parts and
specifically accumulates in vesicles in laticifers. The benzo[c]phenathridine sanguinarine
is found in root tissue. In plant cell cultures of
P. somniferum, accumulation
of sanguinarine can be elicited by addition of methyl jasmonate (Huang and
Kutchan, 2000), but conditions have not been found under which morphine
accumulates. The reason for the absence of morphine in cell culture is not
completely clear, since all of the enzymes for which
in vitro assays have been
developed are also found in cell culture extracts. With availability of several
biosynthetic cDNAs from
P. somniferum, information as to the localization of
selected biosynthetic enzymes and, therefore, the spatial distribution of alkaloid
biosynthesis becomes clearer.
Tyrosine/dopa decarboxylase participates in the very early stages of tetrahydrobenzylisoquinoline
alkaloid biosynthesis. In
P. somniferum, this enzyme is
encoded by a multigene family, which is classified into two groups
tydc1 and
tydc2 (Facchini and De Luca, 1994). From
in situ hybridization experiments,
transcript of
tydc1 was more abundant than
tydc2 in roots, while
tydc2 transcript
was more abundant than
tydc1 transcript in stem (Facchini and De Luca, 1995).
tydc transcript was detected in the metaphloem and protoxylem of vascular bundles in aerial plant parts (Fig. 10.9A and C). This localization is consistent with
latex as the site of morphinan alkaloid accumulation.
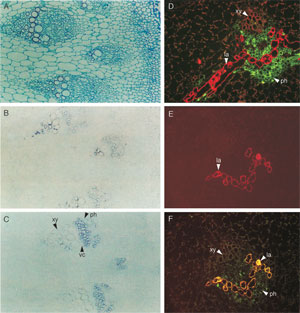 |
| FIGURE 10.9 Localization of tydc (Facchini and
De Luca, 1995), SalAT, COR1, and MLP
(Weid et al., 2004) in stem of P. somniferum.
Panel (A) root cross-section stained with aniline
safranine and
astra blue; (B) in situ hybridization
of tydc1; (C) in situ hybridization of tydc2; (D)
immunolocalization of MLP to laticifers (red
fluorescence) and SalAT to phloem parenchyma
(green fluorescence); (E) immunolocalization of
MLP to laticifers (red fluorescence); and
(F)
coimmunolocalization of MLP and COR1 to
laticifers (yellow fluorescence). Green
fluorescence
indicates COR1 is present also in
phloem parenchyma. xy, xylem; ph, phloem; vc,
vascular cambium;
la, laticifers; tydc,
tyrosine/dopa decarboxylase; SalAT, salutaridinol
7-O-acetyltransferase;
COR1, codeinone
reductase; MLP, major latex protein. |
Immunolocalization of five enzymes of alkaloid biosynthesis, two ofwhich occur
late in the morphine-specific pathway, has been carried out with
P. somniferum.
Morphine biosynthesis is localized to the vascular bundle in capsule, stem, and
root, and involves two different cell types—paranchyma associated with phloem
and laticifer cells (Weid
et al., 2004). Whereas 4'-
omt and SalAT were detected in phloem parenchyma cells, COR1 was abundantly colocalized with major latex
proteins to laticifers (Fig. 10.9D and F). It appears that at a late stage in morphine
formation, at the level of salutaridinol-7-O-acetate or thebaine, biosynthesis moves
out of the phloem parenchyma into the laticifers, the ultimate site of thebaine,
codeine, and morphine accumulation. As for vindoline biosynthesis in
C. roseus (St-Pierre
et al., 1999), more than one cell type is implied in morphine biosynthesis
in
P. somniferum. This spatial distribution of the biosynthetic pathway infers that
transport processes are integral to alkaloid formation. Again, cellular localization
and intermediate transport could be one level of regulation of alkaloid biosynthesis
in
P. somniferum.
Due to commercial importance,
P. somniferum is an alkaloid-producing plant of
choice for metabolic engineering. Promoters will need to be chosen, however, that
will direct transgene expression to the cell types in
P. somniferum in which the
appropriate biosynthetic gene transcripts are expected to occur. As model systems,
plant cell cultures of a multitude of isoquinoline alkaloid-producing
species can be used for metabolic engineering experiments, bypassing in some
instances the complications that arise from multicellular compartmentation in
differentiated plants.





