Genetic Engineering of Tetrahydrobenzylisoquinoline Alkaloid Biosynthetic Pathways
Attempts at engineering plant cell cultures and differentiated plants that produce
isoquinoline alkaloids using cDNAs from tetrahydrobenzylisoquinoline-derived
alkaloid biosynthesis have been hampered by the difficulties associated with
establishing transformation and regeneration protocols for each new species.
Just the
same, there are selected successes to be reported.
One of the first introductions of an isoquinoline alkaloid biosynthetic gene into
an isoquinoline alkaloid-producing plant was the
Agrobacterium-mediated transformation
of the
C. japonica 9-
omt under transcriptional of the CaMV 35S promoter
into
C. japonica cell cultures (Sato
et al., 2001). Ectopic expression of 9-
omt in a high
berberine-producing cell culture resulted in a 15% increase in berberine and
columbamine. Likewise, the
same construct was introduced into
E. californica seedling segments from which transgenic cell cultures were derived.
E. californica produces benzo[c]phenanthridine alkaloids rather than berberine alkaloids. Introduction
of the 9-
omt cDNA resulted in the accumulation of columbamine (not
normally present in
E. californica) and a reduction in the level of the
E. californica native alkaloid sanguinarine. Ectopic expression of 9-
omt successfully introduced
a new branch point in the
E. californica isoquinoline alkaloid pathway and redirected
(S)-scoulerine away from benzo[c]phenanthridine alkaloid biosynthesis into
berberine alkaloid formation.
Root cultures of
E. californica have been engineered with
bbe1 and
cyp80b1
under transcriptional control of the CaMV 35S promoter by
Agrobacterium rhizogenes-mediated transformation (Park
et al., 2002, 2003). Transgenic root cultures containing either antisense
bbe1 or antisense
cyp80b1 accumulated lower
levels of benzo[c]phenanthridine alkaloids compared to controls, whereas transgenic
root cultures that overexpressed
bbe1 showed increased accumulation of
benzo[c]phenanthridine alkaloids compared to controls. These types of experiments
demonstrate that manipulation of alkaloid biosynthetic pathways is possible,
but illustrate that intact transgenic plants are difficult to obtain.
There are several reports in the literature of the transformation and
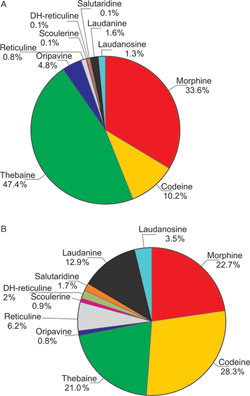 |
| FIGURE 10.10 Benzylisoquinoline
alkaloid quantitation in latex from
(A) untransformed P. somniferum and
(B) transgenic anti-bbe1 T2 plants
(Frick et al., 2004). The data are
presented in
percentages
(µg alkaloid/100 µg soluble protein in
latex). The values given in (A) are the
mean of
individual analyses of 29 plants. |
regeneration of
P. somniferum. The earliest reports of regeneration of
P. somniferum plants
from cell culture preceeded the transformation work (Wakhlu and Bajwa, 1986).
Transformation of
P. somniferum with
A. rhizogenes appeared shortly thereafter
(Williams and Ellis, 1993; Yoshimatsu and Shimomura, 1992). The first report of
Agrobacterium-mediated transformation of
P. somniferum cell suspension cultures
in which an
Arabidopsis thaliana sam1 trangene was introduced occurred in 1997
(Belny
et al., 1997). The first report of
Agrobacterium-mediated transformation with
subsequent regeneration of
P. somniferum appeared in 1999 (Larkin
et al., 1999).
A second report appeared in 2000 (Park and Facchini, 2000). Attempts at metabolic
engineering of alkaloid biosynthesis in
P. somniferum are currently being
made. The known genes of tetrahydrobenzylisoquinoline-derived alkaloid formation
in
P. somniferum have been reintroduced into a highly inbred, elite Tasmanian
variety in the sense and antisense orientation. Several hundred transgenic plants
have been produced by
Agrobacterium-mediated transformation (S. Frick,
P. J. Larkin, and T. M. Kutchan, unpublished data). The first set of transgenic
plants for which the analyses are complete are those that contained the
bbe1 gene
in an antisense orientation under transcriptional control of the S4S4 promoter
(Frick
et al., 2004). These experiments were designed to reduce flow of the central
intermediate (S)-reticuline into the sanguinarine pathway. A complex picture of
ratios of tetrahydrobenzylisoquinoline intermediate alkaloids emerged from these
experiments, but morphinan levels did not increase (Fig. 10.10). Preliminary
analyses of plants that contain various other transgenes indicate that it is, however,
possible to manipulate morphine levels.
P. somniferum has tremendous
potential for alkaloid engineering because the plant is the commercial source of
pharmaceutically important morphinan alkaloids and the transformation and
regeneration, although still a slow process, has been established.





