Monoterpenoid Indole Alkaloid Biosynthesis
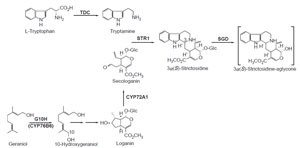 |
| FIGURE 10.1 Schematic representation of the
biosynthetic pathway leading from L-tryptophan
and geraniol to the hypothetical intermediate
3α(S)-strictosidine-aglycone. tdc, tryptophan
decarboxylase; str1, strictosidine synthase;
G10H (CYP76B6), geraniol 10-hydroxylase;
cyp72a1,
secologanin synthase; SGD,
strictosidine glucosidase. |
On the pathway leading from L-tryptophan and geraniol to the central monoterpenoid
indole alkaloid intermediate 3α(S)-strictosidine aglycone, the cDNAs
encoding five biosynthetic enzymes have been described (Fig. 10.1). These are
tydc (tryptophan decarboxylase) (De Luca
et al., 1989),
g10h or
cyp76b6 (geraniol
10-hydroxylase) (Collu
et al., 2001),
cyp72a1 (secologanin synthase) (Irmler
et al., 2000),
str1 (strictosidine synthase) (Kutchan
et al., 1988, 1989; Mcnight
et al., 1990),
and
sgd (strictosidine glucosidase) (Geerlings
et al., 2000; Gerasimenko
et al., 2002). 3α(S)-Strictosidine aglycone is an unstable intermediate that can be transformed
into a number of chemical structures, which then lead into specific monoterpenoid
indole alkaloid pathways. Along the biosynthetic pathway that leads from
tabersonine to vindoline, three cDNAs encoding biosynthetic enzymes have been
identified (Fig. 10.2). The cytochrome
P450-dependent monooxygenase gene
cyp71d12 encodes tabersonine 16-hydroxylase (Schröder
et al., 1999; St-Pierre
and De Luca, 1995). Desacetoxyvindoline is hydroxylated at the 4-
position by
the oxoglutarate-dependent dioxygenase desacetoxyvindoline–4-hydroxylase
(encoded by
d4h) (De Carolis and De Luca, 1993; Vazquez-Flota
et al., 1997).
Finally, deacetylvindoline is acetyla
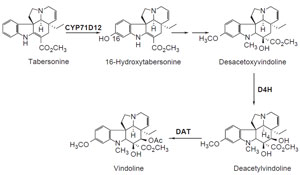 |
| FIGURE 10.2 Schematic representation of the
biosynthetic pathway leading from tabersonine to
vindoline. CYP71D12, tabersonine
16-hydroxylase; d4h, desacetoxyvindoline
4-hydroxlyase; dat,
deacetylvindoline
4-O-acetyltransferase. |
ted to vindoline by acetylcoenzyme A: deacetylvindoline
4-O-acetyltransferase, the gene product of
dat (Fahn
et al., 1985;
Power
et al., 1990; St-Pierre
et al., 1998).
Although not specific to monoterpenoid indole alkaloid biosynthesis, the
nonmevalonate pathway has been shown to be the biosynthetic route to
loganin and secologanin (Contin
et al., 1998; Eichinger
et al., 1999), and three
cDNAs encoding 1-deoxy-D-xylulose 5-phosphate synthase, 1-deoxy-D-xylulose
5-phosphate reductoisomerase, and 2C-methyl-D-erythritol 2,4-cyclodiphosphate
synthase of this pathway have been isolated from
C. roseus (Chahed
et al., 2000;
Veau
et al., 2000).
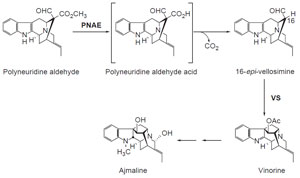 |
| FIGURE 10.3 Schematic representation of the
biosynthetic pathway leading from polyneuridine
aldehyde to ajmaline. PNAE, polyneuridine
aldehyde esterase; VS, vinorine synthase. |
Progress has also been made in identifying cDNAs encoding enzymes of
monoterpenoid indole alkaloid biosynthesis in
R. serpentina (Fig. 10.3). Two
cDNAs involved in the transformation of the sarpagan alkaloid polyneuridine
aldehyde into the ajmalan-type alkaloid ajmaline have been characterized:
pnae encoding polyneuridine aldehyde esterase (Dogru
et al., 2000; Pfitzner and
Stöckigt, 1983) that converts polyneuridine aldehyde into epivellosimine which is then rearranged and acetylated to vinorine by vinorine synthase encoded by
the
vs gene (Bayer
et al., 2004).
With the current collection of cDNAs encoding enzymes of monoterpenoid
indole alkaloid biosynthesis, progress has also been made with respect to our
understanding of the regulation of this biosynthesis. The first topic to be covered
here is the cellular localization of monoterpenoid indole alkaloid biosynthesis.







