Tropane Alkaloid Biosynthesis
The biosynthetic building blocks leading to the tropane alkaloids are theamino acids
L-arginine and L-phenylalanine (Fig. 10.11). On the pathway leading from L-arginine
to the N-methylpyrrolinium ion, the enzyme putrescine N-methyltransferase
(
pmt) has been well characterized at the enzymatic and molecular genetic levels
(Hibi
et al., 1992). Several
pmt genes have been isolated from
Nicotiana tabacum,
Atropa belladonna, Hyoscyamus niger, N. sylvestris, and
N. attenuate (Hibi
et al., 1994;
Shoji
et al., 2000; Suzuki
et al., 1999a; Winz and Baldwin, 2001). Further along the biosynthetic pathway to tropane alkaloids, tropinone is reduced by tropinone
reductase I (
tr-I) to tropine.
tr-I specifically reduces the tropinone 3-keto moiety
to the 3α-hydroxyl group of tropine, the biosynthetic precursor of hyoscyamine
and scopolamine (Hashimoto
et al., 1992; Koelen and Gross, 1982). Tropinone
reductase II (
tr-II) reduces the 3-keto group of tropinone to the 3β-hydroxy
moiety of pseudotropine, which serves as precursor to the calistegins (Dräger and
Schaal, 1994). The gene
tr-I has been characterized from datura stramonium and
H. niger (Nakajima
et al., 1993, 1999). The gene
tr-II is known from
D. stramonium,
H. niger, and
Solanum tuberosum (Keiner
et al., 2002; Nakajima
et al., 1993, 1999). The
final gene fr
omthe scopolamine biosynthetic pathway that has been
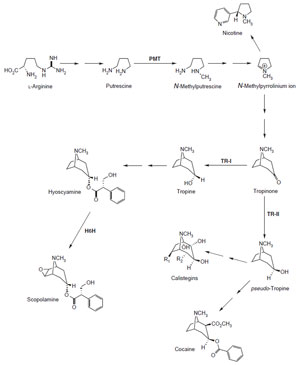 |
| FIGURE 10.11 Schematic representation of the
biosynthetic pathway leading from L-arginine to
nicotine, scopolamine, calistegins, and cocaine.
pmt, putrescineN-methyltransferase; tr-I,
tropinone
reductase I; tr-II, tropinone reductase
II; h6h, hyoscyamine 6β-hydroxylase. |
identified is
h6h encoding hyoscyamine 6β-hydroxylase (Hashimoto and Yamada, 1986). This
2-oxoglutarate-dependent dioxygenase is bifunctional, catalyzing both the monooxygenation
of hyoscyamine to 6b-hydroxyhyoscyamine and the subsequent
epoxi
dation to scopolamine. This gene has been characterized from
H. niger and
A. belladonna (Matsuda
et al., 1991; Suzuki
et al., 1999b).
With the cloning of the genes
pmt,
tr-I,
tr-II, and
h6h, our understanding of
the cellular localization of solanaceous alkaloid biosynthesis has also advanced.
The current view is discussed in the following sections.