Direct Stain and Indirect Stain
Bacterial morphology (form and structure) may be examined in 2 ways:
- By observing living unstained organisms (wet mount), or
- By observing killed, stained organisms.
- See greater contrast between the organism and the background
- Differentiate various morphological types (by shape, arrangement, Gramreaction, etc.)
- Observe certain structures (flagella, capsules, endospores, etc.).
Before staining bacteria, you must first understand how to “fix” the organisms to the glass slide. If the preparation is not fixed, the organisms will be washed off the slide during staining. A simple method is that of air drying and heat fixing. The organisms are heat fixed by passing an air-dried smear of the organisms through the flame of a gas burner. The heat coagulates the organisms’ proteins, causing the bacteria to stick to the slide.
The procedure for heat fixation is as follows:
- If the culture is taken from an agar medium:
- Using the dropper bottle of distilled water found in your staining rack, place ½ drop of water on a clean slide by touching the dropper to the slide.
- Aseptically remove a small amount of the culture from the agar surface and touch it several times to the drop of water until it turns cloudy.
- Burn the remaining bacteria off the loop. (If too much culture is added to the water, you will not see stained individual bacteria.)
- Using the loop, spread the suspension over the entire slide to form a thin film.
- Allow this thin suspension to completely air dry.
- Pass the slide (film-side up) through the flame of the Bunsen burner 3 or 4 times to heat-fix.
Caution: Too much heat might distort the organism and, in the case of the Gram stain, may cause Gram-positive organisms to stain Gramnegatively. The slide should feel very warm, but not too hot to hold. - If the organism is taken from a broth culture:
- Aseptically place 2 or 3 loops of the culture on a clean slide. Do not use water.
- Using the loop, spread the suspension over the entire slide to form a thin film.
- Allow this thin suspension to completely air dry.
- Pass the slide (film-side up) through the flame of the bunsen burner 3 or 4 times to heat-fix.
Dyes or stains may be divided into 2 groups: basic and acidic. If the color portion of the dye resides in the positive ion, as in the above case, it is called a basic dye (examples: methylene blue, crystal violet, safranin). If the color portion is in the negatively charged ion, it is called an acidic dye (examples: nigrosin, congo red).
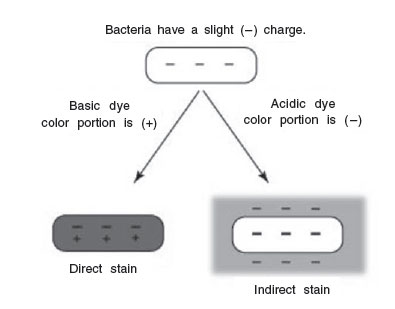 |
| Figure 25 Direct staining and indirect staining. |
Because of their chemical nature, the cytoplasm of all bacterial cells have a slight negative charge when growing in a medium of near-neutral pH. Therefore, when using a basic dye, the positively charged color portion of the stain combines with the negatively charged bacterial cytoplasm (opposite charges attract) and the organism becomes directly stained.
An acidic dye, due to its chemical nature, reacts differently. Since the color portion of the dye is on the negative ion, it will not readily combine with the negatively charged bacterial cytoplasm (like charges repel). Instead, it forms a deposit around the organism, leaving the organism itself colorless. Since the organism is seen indirectly, this type of staining is called indirect or negative, and is used to get a more accurate view of bacterial sizes, shapes, and arrangements.
Try both direct and indirect stains of several microorganisms.
Direct Stain using a Basic Dye
In direct staining, the positively charged color portion of the basic dye combines with the negatively charged bacterium, and the organism becomes directly stained.
Organisms
Your pure cultures of Staphylococcus epidermidis (coccus with staphylococcus arrangement) or Micrococcus luteus (coccus with a tetrad or a sarcina arrangement) and Escherichia coli (small bacillus) or Enterobacter aerogenes (small bacillus).
Procedure
- Heat-fix a smear of either Escherichia coli or Enterobacter aerogenes as follows:
- Using the dropper bottle of distilled water found in your staining rack, place a small drop of water on a clean slide by touching the dropper to the slide.
- Aseptically remove a small amount of the culture from the agar surface
- Burn the remaining bacteria off the loop. (If too much culture is added to the water, you will not see stained individual bacteria.)
- Using the loop, spread the suspension over the entire slide to form a thin film.
- Allow this thin suspension to completely air dry.
- Pass the slide (film-side up) through the flame of the bunsen burner 3 or 4 times to heat-fix.
- Place the slide on a staining tray and cover the entire film with safranin. Stain for 1 minute.
- Pick up the slide by one end and hold it at an angle over the staining tray. Using the wash bottle on the bench top, gently wash off the excess safranin from the slide. Also wash off any stain that got on the bottom of the slide.
- Use a book of blotting paper to blot the slide dry. Observe using oil immersion microscopy.
- Prepare a second direct, this time using either Staphylococcus epidermidis or Micrococcus luteus as the organism.
- Heat-fix a smear of the Micrococcus luteus or Staphylococcus epidermidis by following the directions under step 1.
- Stain with methylene blue for 1 minute.
- Wash off the excess methylene blue with water.
- Blot dry and observe using oil immersion microscopy.
- Prepare a third slide of the normal flora and cells of your mouth.
- Using a sterile cotton swab, vigorously scrape the inside of your mouth and gums.
- Rub the swab over the slide (do not use water), air dry, and heat-fix.
- Stain with crystal violet for 30 seconds.
- Wash off the excess crystal violet with water.
- Blot dry and observe. Find epithelial cells using your 10X objective, center them in the field, and witch to oil immersion to observe the normal flora bacteria on and around your epithelial cells.
Indirect Stain using an Acidic Dye
In negative staining, the negatively charged color portion of the acidic dye is repelled by the negatively charged bacterial cell. Therefore, the background will be stained and the cell will remain colorless.
Organism
Your pure culture of Staphylococcus epidermidis or Micrococcus luteus.
Procedure
- Place a small drop of nigrosin on a clean slide.
- Aseptically add a small amount of Staphylococcus epidermidis or Micrococcus luteus to the dye and mix gently with the loopUsing the edge of another slide, spread the mixture with varying pressure across the slide so that there are alternating light and dark areas. Make sure the dye is not too thick or you will not see the bacteria!
- Let the film of dyed bacteria air dry completely on the slide. Do not heatfix and do not wash off the dye.
- Observe using oil immersion microscopy. Find an area that has neither too much nor too little dye (an area that appears light purple where the light comes through the slide). If the dye is too thick, not enough light will pass through; if the dye is too thin, the background will be too light for sufficient contrast.
Results
Make drawings of your 3 direct stain preparations and your indirect stain preparation.
Performance Objectives
Introduction to Staining
Describe the procedure for heat fixation
- Define the following: acidic dye, basic dye, direct stain, and indirect stain.
- Describe in chemical and physical terms the principle behind direct staining and the principle behind indirect staining.
Direct Staining
Procedure
- Transfer a small number of bacteria from an agar surface or a broth culture to a glass slide and heat-fix the preparation.
- Prepare a direct stain when given all the necessary materials.
Results
Recognize a direct stain preparation when it is observed through a microscope, and describe the shape and arrangement of the organism.
Indirect Staining Procedure
- Perform an indirect stain when given all the necessary materials.
- Explain why the dye is not washed off when doing an indirect stain.
Results
Recognize an indirect stain preparation when it is observed through a microscope, and describe the shape and arrangement of the organism.




