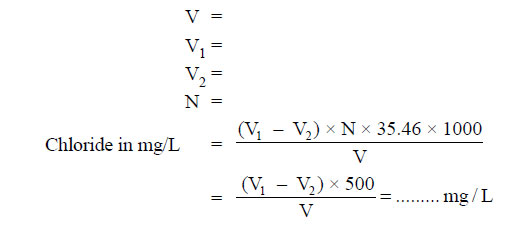Determination of Chloride in Water
To determine the amount of chloride (in the form of Cl–) present in the given water sample by Mohr’s method.
Principle
If water containing chlorides is titrated with silver nitrate solution, chlorides are precipitated as white silver chloride. Potassium chromate is used as indicator, which supplies chromate ions. As the concentration of chloride ions approaches extinction, silver ion concentration increases to a level at which reddish brown precipitate of silver chromate is formed indicating the end point.
- Burette
- Pipettes
- Erlenmeyer flasks
- Measuring cylinder
Reagents (click to check the preparation of reagents)
- Chloride free distilled water.
- Standard silver nitrate solution (0.0141N)
- Potassium chromate indicator.
- Acid or alkali for adjusting pH.
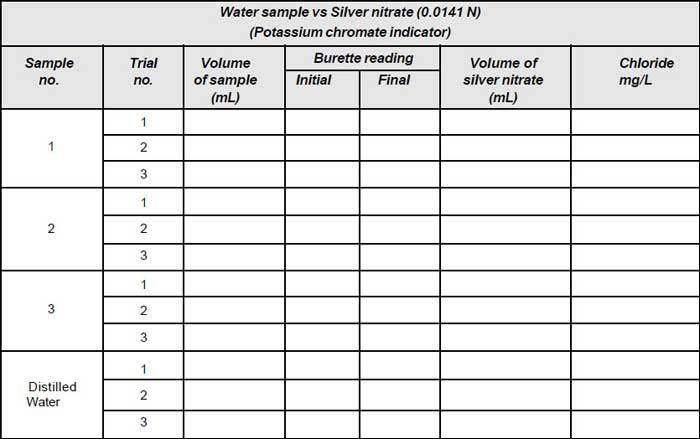
- Take 50mL of sample (V) and dilute to 100mL.
- If the sample is coloured add 3mL of aluminium hydroxide, shake well; allow to settle, filter, wash and collect filtrate.
- Sample is brought to pH 7-8 by adding acid or alkali as required.
- Add 1mL of indicator (Potassium chromate).
- Titrate the solution against standard silver nitrate solution until a reddish brown precipitate is obtained.
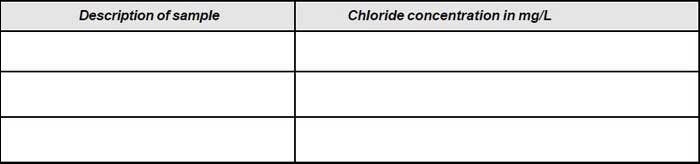
Note down the volume (V1). - Repeat the procedure for blank and note down the volume (V2).