Determination of Nitrite Nitrogen
To determine the nitrite nitrogen of the given sample of water.
Principle
The nitrite concentration is determined through the formation of a reddish-purple azo dye produced at pH 2.0–2.5 by the coupling of diazotised sulphanilic acid with N-(1-naphthyl)-ethylenediamine dihydrochloride.
- Spectrophotometer
Reagents (click to check the preparation of reagents)
- Sulphanilamide reagent
- N-(1-naphthyl)-ethylenediamine dihydrochloride solution
- Hydrochloric acid (1+3)
- Stock nitrite solution
- Standard nitrite solution
- To 50 ml clear sample neutralised to pH 7, add 1ml sulphanilamide solution.
- Allow the reagent to react for a period of 2–8 minute.
- Then add 0.1ml of 1-naphthyl ethylenediamine solutions and mix immediately.
- Measure the absorbance of the solution after 10 minute at 543 nm at 1 cm light path.
- Prepare standard calibration curve as in any other case.
- By noting the absorbance of an unknown sample, the concentration of nitrate can be determined
Calculation
| Nitrite N in mg/L = | mg Nitrite N |
| mL of sample |
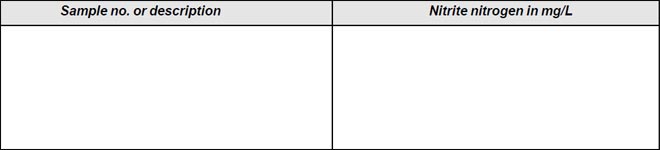
Observation
The observation is presented in Tables A and B respectively
Table A: Observation for calibration
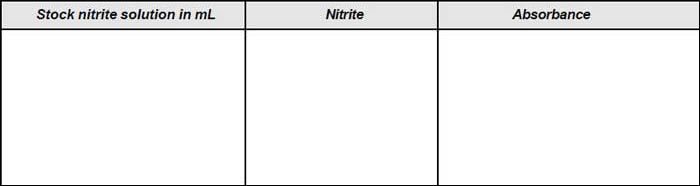
Table B:

Results