Current Sources/Markets for Specialty Allyl/Propenyl Phenols
The major current source of allyl/propenyl phenols is clove oil, which generally
contains eugenol (
33, 75–85%), eugenyl acetate (35, 8–15%), and other minor
components. The oil is a product of the clove tree,
Syzygium aromaticum [
Eugenia
caryophyllata], and is mainly produced in Madagascar, Tanzania (Zanzibar and
Pemba Islands), as well as in Indonesia. Worldwide production of this oil approximates
2000 ton year
-1 (Bauer
et al., 2001), with a gross market value of approximately
US$ 30–US$ 70 million per annum through its applications in flavors,
fragrances, and antibacterials alone (George Uhe Company, 2006). This market
size is, however, largely dictated by (
1) the labor-intensive gathering of cloves,
(
2) the costs associated with obtaining the oil, and (
3) limited growth/production
capabilities. Therefore, a potential exists for the expansion of the source(s) of these
molecules through the use of new (bio)technologies.
Eugenol (
33) and its natural derivative, eugenyl acetate (
35), are widely used in
the perfumery and flavor industries, being considered by the Food and Drug
Administration (FDA) as Generally Recognized As Safe (‘‘GRAS’’) for use as food
additives. For example, eugenol (
33) is responsible for the majority of clove-like
flavors in beverages, ice cream, baked goods, and candy (Maralhas
et al., 2006),
whereas methyleugenol (
34) is a flavoring ingredient in ice cream, candy, and cola
soft drinks (Smith
et al., 2001; U.S. Environmental Protection Agency, 2006a).
Eugenol (
33) is also present in kretek (clove) cigarettes (usually as 40% ground
cloves, 60% tobacco) and is used as an industrial source of isoeugenol (
39) (by
alkaline isomerization) and methyleugenol (
34). Because of its excellent analgesic
and antibacterial properties, eugenol (
33) has long been employed in dentistry in
combination with zinc oxide forming a polymerized eugenol cement, which is
then used for surgical dressings, temporary fillings, pulp capping agents, and
cavity liners (Skinner, 1940; Weinberg
et al., 1972). Isoeugenol (
39) is widely used
as a flavor and fragrance additive in baked goods, beverages, chewing gum, and
personal products such as perfumes and soaps (Badger
et al., 2002). Isoeugenol
(
39) also used to be the main source for the industrial production of vanillin
(
40, Fig. 13.9), but the latter is now more commonly derived either from
the petrochemical guaiacol (
41) or from by-products of the pulp/paper (lignin)
industry (Hocking, 1997).
Chemically related products, anethole (
38) and methylchavicol (
32), on the
other hand, are currently isolated as minor by-products (1–2%) of crude sulfate
turpentine (CST) processing (The Flavor and Fragrance High Production Volume,
2002; 2005). This requires a lengthy isolation process of fractional distillation starting
from CST, and fractional crystallization from the semipurified terpenoid/phenolic mixtures thus produced.Most of themethylchavicol (
32) obtained in this way is then
subjected to alkaline isomerization to generate
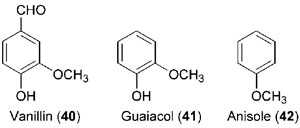 |
| FIGURE 13.9 Structures of vanillin (40), guaiacol
(41), and anisole (42). |
more anethole (
38), although chemical
synthesis from anisole (42, Fig. 13.9) using propionyl chloride or propionaldehyde
can also be used. Although CST accounted for 97% of total turpentine
production in the United States in 1990, steadily increasing from 27.4% in 1950, the
total production of turpentine declined to approximately 20.7 million gallons in 1999
(Haneke, 2002) [containing an estimated 680 and 740 tons methylchavicol (
32) and
anethole (
38), respectively]. As wages increased, the labor-intensive production of
turpentine oil became increasingly less competitive economically, and thus much of
the original botanical-derived turpentine was substituted by cheaper petroleumbased
solvents in the intervening years.Anethole (38) finds application as a perfume
in detergents, soaps, and shampoos, and as a flavoring agent in licorice, ice cream,
baked goods, and alcoholic beverages (Newberne
et al., 1999), while methylchavicol
(
32) is added as a component of root beer and anise-type flavors, as well as condiments
and meat seasonings (The Flavor and Fragrance High Production Volume
Consortia, 2005).
Some allyl/propenyl phenols have also recently been approved by the Environmental
Protection Agency (EPA) for use as insecticides, insect repellents, and/
or insect lures, perhaps reflecting the original roles/functions of these natural
products
in planta. Applied as unmodified natural products, they have more
specific and less persistent insecticidal activities and therefore pose reduced
environmental risk relative to the commonly used organophosphates and pyrethroids.
Interestingly, eugenol (
33) is used as an insecticide not only for various
crop plants and ornamentals but also for pets (U.S. Environmental Protection
Agency, 2006b); furthermore, it can be used as an attractant in insect traps specific
for Japanese beetles (U.S. Environmental Protection Agency, 2006b,c). Anise oil,
on the other hand, is approved for use on lawns and ornamentals as a dog and cat
repellant (U.S. Environmental Protection Agency, 2006d), whereas methylchavicol
(
32), when applied to trees, repels bark beetles (for example, the southern pine
beetle) thereby limiting their aggregation and reproduction abilities (U.S.
Environmental Protection Agency, 2006e). Additionally, methyleugenol (
34) is
perhaps the most economically important allyl/propenyl phenol insect attractant,
being in widespread use in insect traps for certain fruit flies, including the
Oriental fruit fly, Mediterranean fruit fly, and solanaceous fly (Jang
et al., 2003;
U.S. Environmental Protection Agency, 2006a). (Fruit flies are one of the most
destructive pests to Hawaii’s agricultural industry, where these low-cost traps are
one of the few environment-friendly recommended suppression techniques.)

