Potential for Allyl/Propenyl Phenols?
We consider that biotechnological approaches utilizing the enzymatic machinery
described above should now be explored to produce allyl and propenyl phenols,
diverting (to a predetermined extent) some of the carbon flow, for example, from
lignin toward these metabolites, while maintaining the resulting plants’ capacities
to grow and function within acceptable boundaries. The resulting cellulosic biomass
could thus become more amenable to pulp/paper manufacture and/or
biofuel production due to the lower lignin contents. Alternatively, oilseed metabolism
could be manipulated to produce these allyl/propenyl phenol substances
in larger amounts. Upon processing and purification, these compounds could
potentially find uses as biofuels, biofuel precursors, flavors/fragrances, and/or
intermediate chemicals (e.g., as monomers for synthetic polymers).
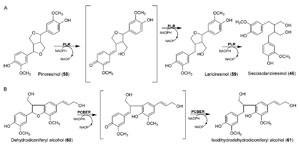 |
| FIGURE 13.17 Reactions catalyzed by
(A) PLR and (B) PCBER. |
Moreover, the ability to routinely biotechnologically modify plants and/or cell
cultures to produce allyl/propenyl phenols, such as chavicol (
31) and eugenol
(
33), now offers the opportunity to consider much larger markets for these products, that is, in addition to expanding the flavor/fragrance/antiseptic/biocidal
markets that currently exist (see above Section 3). For instance, in terms of
the flavor/fragrance market, the natural vanillin market can potentially be
expanded. Today, only about 0.2% of vanillin (
40, Fig. 13.9) used originates
directly from its botanical source, the vanilla bean, where it commands a sales
price of approximately US$ 4000 kg
-1 as a natural product. The bulk is semisynthetic,
being chemically synthesized either from the petrochemical-derived guaiacol
(
41) or from pulp/paper lignin-derivatives (i.e., technical lignins). However,
the vanillin (
40) produced in this way commands a price of only US$ 12 kg
-1 because it is not ‘‘natural.’’
Driven by consumer preferences toward truly ‘‘natural’’ food products, food
additives, and pharmaceuticals, several biocatalytic processes for production of
plant-derived metabolites using microbes and plant cell cultures have been developed
and patented in
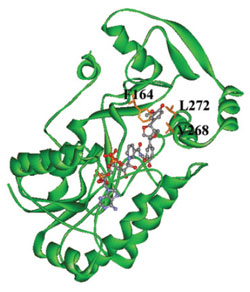 |
| FIGURE 13.18 Schematic representation
of the crystal structure of TpPLR1 with
NADPH and
(-)-pinoresinol (58).
Source: Reprinted from Min et al.
(2003). |
recent years (Berger, 1991; Krings and Berger, 1998; Longo
and Sanromán, 2006; Priefert
et al., 2001; Rabenhorst and Hopp, 1991; Schrader
et al., 2004; Shimoni
et al., 2000; Yoshimoto
et al., 1990). This enthusiasm has been
fueled by the promise to generate transgenic cultures with increased efficacy for
production of target metabolites. This approach also apparently offers economic
advantages over conventional chemical syntheses, including better stereospecificity
in product formation and lower amounts of waste products being generated
upon processing (Schoemaker
et al., 2003). In particular, production of flavors via
biotechnological processes offers an additional economic advantage since, unlike
their chemically prepared counterparts, the resulting products can be marketed as
‘‘natural’’ under current US and EU legislation (The European Commission, 1991;
Food and Drug Administration, 2006; Lesage-Meessen
et al., 1996; Shimoni
et al.,
2000). In this regard, successful microbial production of vanillin (40) from various
precursors, such as eugenol (
33) and isoeugenol (
39), has been reported recently,
being achieved at relatively high substrate concentrations (Krings and Berger,
1998; Longo and Sanroma´n, 2006; Priefert
et al., 2001; Rabenhorst and Hopp, 1991;
Schrader
et al., 2004; Shimoni
et al., 2000);
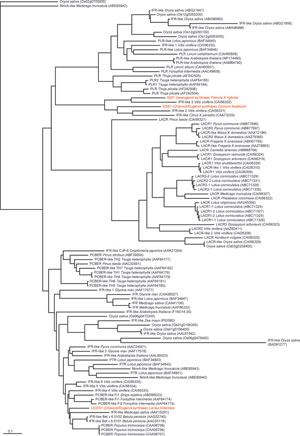 |
| FIGURE 13.19 Currently annotated phylogenetic
analysis of several PIp-reductase homologues
from different plant species, with relevant
homologues in basil (ObEGS1), petunia
(PhIGS1), and
creosote bush (LtCES1)
highlighted. IFR, isoflavone reductase; LACR,
leucoanthocyanidin reductase;
NmrA, nitrogen
metabolite repression regulator; PCBER,
phenylcoumaran-benzylic ether reductase;
PLR,
pinoresinol-lariciresinol reductase; PTR,
pterocarpan reductase. Sequences were
obtained
from the NCBI database and filtered for <0.75
sequence difference, ClustalW-aligned,
and
subjected to neighbor-joining phylogenetic
analysis using PHYLIP (Felsenstein, 1993). |
for example, transformation of
isoeugenol (
39) (20 g/L) using a strain of
Serratia marcescens led to vanillin (
40)
accumulation (3.8 g/L). An enzymatic process for conversion of isoeugenol (
39) into vanillin (
40) using a ligno stilbene-α,β-dioxygenase from a
Pseudomonas
paucimobilis strain has also been patented (Yoshimoto
et al., 1990). Thus, the
technologies are now apparently in hand to permit formation of natural vanillin
(
40) through established microbial and genetic manipulations in either plants
and/or plant/bacterial cell cultures.
Much larger anticipated potential markets for the allyl/propenyl phenols
include the industrial polymers and biofuel/biodiesel. Regarding polymer applications,
the expected worldwide production of polystyrenes alone was approximately
25 million metric tons in 2006, representing sales of US$ 31 billion. Allyl/
propenyl phenols can be converted into functionalized polystyrene derivatives,
and an increased supply creates the potential for their massive usage as intermediate
(monomer) chemicals in industrial polymers. Currently, existing applications
include eugenol- (
33) based polymers, which are widely used in dentistry in
zinc oxide impression pastes applied as surgical dressings and temporary
cements (Skinner, 1940; Weinberg
et al., 1972), as well as specialty modifying
(i.e., coating) agents in analytical electrodes (Ciszewski and Milczarek, 1998,
1999, 2001, 2003; Rahim
et al., 2004).
The functionalized β-methylstyrenes anethole (
38) and isoeugenol (
39) can be
converted into polymers of several thousand dalton (Bywater, 1963). However,
the potential of such conversions has been studied only to a limited extent relative
to their vinyl analogues (i.e., styrene and derivatives thereof), in part due to
limited supply/availability and because propenylbenzenes do not apparently
undergo as efficient free radical polymerization reactions as styrenes—even
though their electronic configuration is such that a radical intermediate can also
be stabilized by the aromatic ring (Alexander
et al., 1981). The most efficient
polymerization initiators described thus far for propenylbenzene derivatives are
Lewis acids, particularly AlCl
3, SnCl
4, and BF
3 (Alexander
et al., 1981; Cerrai
et al.,
1969a,b; Secci and Mameli, 1956).
In general, the steric factors on the monomers define, to a large extent, both
polymerization rates and molecular weights of the resulting polymers, with anethole
(
38), for example, being more reactive than isoeugenol (
39) (Alexander
et al.,
1981). Polymerization reactions can proceed through a conventional 1,2-chain
formation, similar to styrene, with the propagating species being a Lewis acidinduced
carbocation that is added to the double bond of another monomer.
This
results in a polymer backbone composed of the carbons 7 and 8 of the original
monomers. The molecular weights of the resulting polymers are higher at lower
temperatures and, in the case of anethole (
38) when polymerized by SnCl4, can
vary from a few thousand up to about 75,000 Da depending on both temperature
and dilution levels (Bywater, 1963; Cerrai
et al., 1969a; Secci and Mameli, 1956).
For isoeugenol (
39), the phenolic oxygen moiety also participates in the polymerization
reactions, thereby increasing the structural complexity of the resulting
polymer(s) so formed (Fig. 13.20A) (Evliya and Olcay, 1974). Additionally, allylphenols,
such as methylchavicol (
32) and eugenol (
33), can form mixed polymers,
resulting from the partial rearrangement of the side-chain double bond upon
carbocation formation prior to attachment to the polymer chain (Cihaner
et al.,
2001; Kennedy, 1964) (Fig. 13.20B).
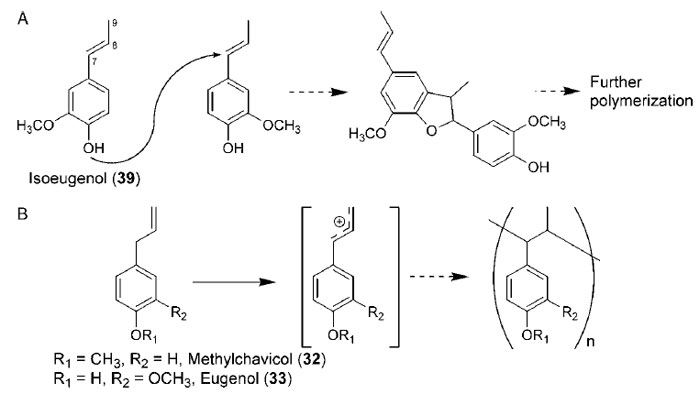 |
| FIGURE 13.20 Polymerization of (A) isoeugenol (39) via furanocoumaran intermediacy and
(B) methylchavicol
(32) and eugenol (33) through rearrangement prior to polymerization. |
In terms of their potential uses as biofuels, it is noteworthy that ~100 billion
gallons of gasoline fuel were consumed in the United States in 2005. In addition,
the annual consumption of diesel fuel in 2000, including highway diesel, farms,
electric power, railroad, fuel oil (residential, commercial, and heating), and kerosene,
totaled approximately 57.1 billion gallons. As a measure of the potential
scale of production of biodiesel (from vegetable oils, consisting mainly of fatty
acid esters), Peterson (1995) recently estimated that if
all harvested cropland (~363
million acres) in the United States was dedicated exclusively to rapeseed (oilseed)
production, then approximately 36.3 billion gallons (assuming 100 gallon/acre) of
vegetable oil could be obtained annually. [Note that at present there are approximately
27 billion gallons of vegetable oil produced worldwide annually (Peterson,
1995)]. Yields for other plant species, modified to concurrently synthesize chavicol
(
31), methylchavicol (
32), or eugenol (
33), now need to be determined to establish
to what extent these productivity numbers can be increased through (for instance)
whole plant utilization.
Additionally, if one considers the annual pulp and paper production in the
United States (~120 million metric tons/year, 1997 figures), the potential also
exists to divert some part of the production of lignins/heartwood lignans and
other phenylpropanoid derivatives in commercially important woody plant species
away from their natural biosynthetic pathways, that is, to afford allyl/propenyl
phenols, etc. In principle, the lignin/lignan substances currently produced
annually as by-products of pulp/paper industries (more than 50 million tons)
could instead be converted to approximately 15 billion gallons allyl/propenyl
phenols per annum, if fully converted. Nevertheless, any reduction in carbon
flow to lignin, or reductions/changes in heartwood-forming constituents, could
represent a significant increase in biofuel production.
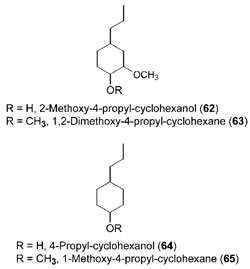 |
| FIGURE 13.21 Cyclohexane derivatives
formed upon catalytic hydrogenation of
the
corresponding allyl/propenyl phenols. |
Allyl and propenyl phenols have relatively high heats of combustion at room
temperature, with values generally being about 70% (per weight) of medium
chain hydrocarbons such as octane and decane. That is, these allyl/propenyl
phenols can potentially generate more energy (per weight) than ethanol. In
terms of other relevant properties, using two examples only for illustrative purposes,
chavicol (
31) has boiling/flash points of 238/102°C at normal atmospheric
pressure and density approximately 1.01 g/cm
3, whereas eugenol (
33) values are
approximately 253/112°C and 1.07 g/cm
3 (at 20°C). Such values are within the
ranges needed for biodiesel/biofuel considerations. Their reported freezing points
are, however, generally between –10°C and room temperature, which would
reduce their potential as liquid biofuels if used exclusively as such in pure liquid
form. This limitation might be circumvented, however, by either blending them
into other fuels, similar to the coconut oils added as biofuels to diesel in the
Philippines (BBC/PRI/WGBH The World, 2007) or through their chemical derivatization
to generate materials of lower freezing point prior to biofuel use (e.g.,
hydrogenation, which may also help reduce pollutant emission upon combustion).
Catalytic hydrogenation of side-chain double bonds of allyl/propenylbenzenes
is readily achieved at atmospheric pressures, whereas reduction of the
aromatic ring typically requires higher temperatures and pressures using traditional
metal catalysts (e.g., supported Pd or Raney Ni). Reduced allyl/propenyl
phenols have already been generated by such catalytic hydrogenation reactions;
for example, 2-methoxy-4-propyl-cyclohexanol (
62, Fig. 13.21) was obtained in
near-quantitative amounts from eugenol (
33) (Maillefer, 1990). Newer catalytic
systems, however, have the exciting potential to dramatically improve the
reduction conditions, for example, as recently reported for the quantitative hydrogenation of several benzene analogues, at room temperature and atmospheric
hydrogen pressure, using ruthenium-containing methylated cyclodextrin
catalysts (Nowicki
et al., 2006). Thus, this application of biotechnology, if further
explored/developed/applied, offers a potentially important new avenue for
sources of biofuels/bioenergy.









