Molecular Biology of Plant Pathways / Metabolic Engineering of Plant Allyl/Propenyl Phenol and Lignin Pathways
Lignin Formation and Manipulation
Outcomes of the phenylpropanoid (C
6C
3) pathway (Fig. 13.1) include not only the
lignins in woody/nonwoody vascular plants but also, to varying extents, lignans,
flavonoids, coumarins, anthocyanins, as well as allyl and propenyl phenols in
different species. This pathway has attracted much attention since the 1960s, and
the various biochemical steps—including the historical development of this
field—were comprehensively discussed (Lewis
et al., 1999), as were the trends
observed in various genetic manipulations of the monolignol pathway and the
corresponding downstream effects on lignification (Anterola and Lewis, 2002).A common theme that has emerged is the action of several enzymes of the
phenylpropanoid pathway (e.g., hydroxylases,
O-methyltransferases) not on
free hydroxycinnamic acids, as previously believed, but on hydroxycinnamic
esters, alcohols, and aldehydes. [For needed context, lignin assembly mainly
utilizes three
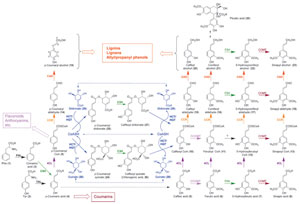 |
| FIGURE 13.1 Current view of the
phenylpropanoid pathway. 4CL,
hydroxycinnamoyl CoA ligases; C3H,
p-coumarate 3-hydroxylase; C4H, cinnamate
4-hydroxylase; CAD, cinnamyl alcohol
dehydrogenases; CCOMT, hydroxycinnamoyl
CoA O-methyltransferases; CCR, cinnamoyl
CoA oxidoreductases;
COMT, caffeic acid
O-methyltransferase; F5H, ferulate
5-hydroxylase; HCT/HQT, hydroxycinnamoyl
shikimate/quinate transferase; PAL,
phenylalanine
ammonia lyase; TAL, tyrosine ammonia lyase. |
monolignols as monomers, namely the phenylpropanoid pathwayderived
p-coumaryl (
19), coniferyl (
21), and sinapyl (
23) alcohols (Fig. 13.1), giving
rise to H (hydroxyphenyl), G (guaiacyl), and S (syringyl) moieties (‘‘units’’),
respectively (Fig. 13.2). The H and G units are present in the lignin of gymnosperms
and ‘‘primitive’’ plants, with angiosperms also containing S components,
the latter being associated with fiber/vessel formation (Lewis
et al., 1999)].





