New Opportunities and Approaches for Renewable Sources of Bioenergy, Biofuels, and Bioproducts?
While the extent to which lignin polymer composition and content can ultimately
be modified and/or reduced is poorly understood, there are alternative biotechnological
opportunities to produce renewable energy biofuels and specialty bioproducts,
which have not been examined yet in detail. That is, there are other
metabolic outcomes of the phenylpropanoid pathway from its entry point phenylalanine
(
1) (depending upon the species) that can include, for example, differential
formation of coumarins, lignans, and flavonoids, as well as allyl/propenyl
phenols (see Fig. 13.1). In particular, allylphenols and propenylphenols, which
differ in the position of their side-chain double bonds, include the high-value
liquids eugenol (
33), estragole [(
32), methylchavicol], and anethole (
38) (Fig. 13.7).
These natural products account for much of the aroma present in specialty
‘‘essential oils’’ of various plant species, such as cloves, tarragon, and anise,
respectively, and are thought to be produced
in planta mainly for defense against
insects and parasites, as well as for attracting pollinators. In addition, most of
these compounds are liquid at room temperature, and their relatively low degree
of oxygenation grants them with high heats of combustion; these are potentially
desirable characteristics when considering their possible utilization as fuels.
Notably, lignins and most lignans, as well as the allyl/propenyl phenols, are all
derived from the same monolignol precursors; thus an approach whereby the
latter are differentially utilized could impact the production/accumulation of
these diverse classes of compounds.
Therefore, biotechnological manipulations of this pathway might be directed
not only toward simply reducing lignin levels but also, perhaps, toward retargeting
carbon toward related metabolic pathways, for example, through redirection
of metabolic (carbon) flux to the production of related phenolic compounds in the
main repositories for plant organic carbon
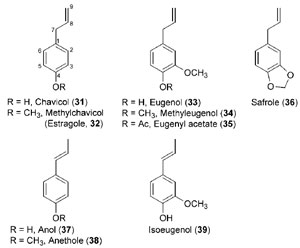 |
| FIGURE 13.7 Selected allylphenols and
propenylphenols. |
storage. The latter could include either
oilseed-bearing structures [e.g., flax (
Linum usitatissimum) seed, Fig. 13.8A] (Ford
et al., 2001; Teoh
et al., 2003) or heartwood-forming tissues of trees [e.g., western
red cedar (
Thuja plicata), Fig. 13.8B] (Fujita
et al., 1999; Kim
et al., 2002). Indeed, it is
these ‘‘repositories’’ that are largely used as plant renewable resources, whether
as sources of (vegetable) oils or for lumber/pulp/paper products. In addition to
the structural lignins, heartwood formation is often accompanied by massive
deposition of non-structural low molecular weight molecules, such as the
monolignol-derived lignan plicatic acid (
30, see Fig. 13.1 for structure) and its
congeners in western red cedar, whose amounts can be approximately 20% of the
overall dry weight (Gardner
et al., 1959, 1960, 1966). Rational optimization/modification
of plant biomass could be done either directly for biofuel/bioenergy/bioproduct
 |
| FIGURE 13.8 (A) Flax (Linum usitatissimum)
oilseed and (B) western red cedar (Thuja plicata)
heartwood. |
generation in specific crops or indirectly as part of (heart)wood processing
for pulp/paper, specialty chemicals, etc. Such considerations have not yet
been explored, although the technologies are now available from previous
research studies (Dinkova-Kostova
et al., 1996; Fujita
et al., 1999; Jiao
et al., 1998;
Kato
et al., 1998; Teoh
et al., 2003; Vassão
et al., 2006b, 2007). For instance, recent
studies have described the formation of some quite well-known phenylpropanoid
pathway monomeric metabolites, namely, the liquid allyl/propenyl phenols,
chavicol (
31), eugenol (
33), and their analogues (
32, 34–39) (Fig. 13.7) (Koeduka
et al., 2006; Vassão
et al., 2006b).
Historically, such allyl/propenyl phenols have commonly been used throughout
the world mainly as flavor/fragrance components present in spices, especially
cloves, with these being largely imported from Tanzania, Madagascar, and Indonesia.
Such material sources are imported simply because it is in these countries
that the plant species accumulating these more unusual metabolites are
cultivated. The recently described biochemical/biotechnological processes
(Koeduka
et al., 2006; Vassão
et al., 2006a,b, 2007) thus offer yet another possibility of diversion of monolignols from either lignin and/or lignan formation in
more commonly utilized woody/nonwoody plant species of, for example,
North America, to afford instead the liquid allyl/propenyl phenol monomers.






