Derivatives of Ectoderm: Nervous System and Nerve Growth
Derivatives of Ectoderm:
Nervous System and Nerve
Growth
The brain, spinal cord, and nearly all the
outer epithelial structures of the body
develop from the primitive ectoderm.
They are among the earliest organs to
appear. Just above the notochord, the
ectoderm thickens to form a neural
plate. The edges of this plate rise up,
fold, and join together at the top to create
an elongated, hollow neural tube.
The neural tube gives rise to most of the
nervous system: anteriorly it enlarges
and differentiates into the brain and cranial
nerves; posteriorly it forms the
spinal cord and spinal motor nerves.
Much of the rest of the peripheral nervous
system is derived from neural
crest cells, which pinch off from the
neural tube before it closes (Figure 8-
25). Among the multitude of different
cell types and structures that originate
with the neural crest are portions of the
cranial nerves, pigment cells, cartilage
and bone of most of the skull (including
the jaws), ganglia of the autonomic nervous
system, medulla of the adrenal
gland, and contributions to several other
endocrine glands. Neural crest tissue is
unique to vertebrates and was probably
of prime importance in the evolution of
the vertebrate head and jaws.
How are the billions of nerve axons in the body formed? What directs their growth? Biologists were intrigued with these questions, which seemed to have no easy solutions. Since a single nerve axon may be more than a meter in length (for example, motor nerves running from the spinal cord to the toes), it seemed impossible that a single cell could reach out so far. One hypothesis was that numerous neural cells joined together in a chain to form an axon. It was alternatively suggested that an axon grew from a series of preformed protoplasmic bridges along its route. The answer had to await the development of one of the most powerful tools available to biologists, the cell culture technique.
In 1907 embryologist Ross G. Harrison
discovered that he could culture
living neuroblasts (embryonic nerve
cells) for weeks outside the body by
placing them in a drop of frog lymph
hung from the underside of a cover slip.
Watching nerves grow for periods of
days, he saw that each axon was the
outgrowth of a single cell. As the axon
extended outward, materials for growth
flowed down the axon center to the
growing tip (growth cone) where they
were incorporated into new protoplasm
(Figure 8-26).
The second question—what directs nerve growth—has taken longer to unravel. An idea held well into the 1940s was that nerve growth is a random, diffuse process. A major hypothesis proposed that the nervous system developed as an equipotential network, or blank slate, that later would be shaped by usage into a functional system. The nervous system just seemed too incredibly complex for us to imagine that nerve fibers could find their way selectively to so many predetermined destinations. Yet it appears that this is exactly what they do! Research with invertebrate nervous systems indicated that each of the billions of nerve cell axons acquires a distinct identity that in some way directs it along a specific pathway to its destination. Many years ago Harrison observed that a growing nerve axon terminated in a growth cone, from which extend numerous tiny threadlike pseudopo-dial processes (filopodia) (Figure 8-26). Recent research has shown that the growth cone is steered by an array of guidance molecules secreted along the pathway and by the axon’s target. This chemical guidance system, which must, of course, be genetically directed, is just one example of the amazing precision that characterizes the entire process of differentiation.
The tissue culture technique developed by Ross G. Harrison is now used extensively by scientists in all fields of active biomedical research, not just by developmental biologists. The great impact of the technique has been felt only in recent years. Harrison was twice considered for the Nobel Prize (1917 and 1933), but he failed ever to receive the award because, ironically, the tissue culture method was then believed to be “of rather limited value.”
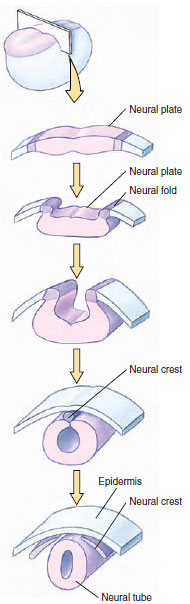 |
| Figure 8-25 Development of the neural tube and neural crest cells from the neural plate ectoderm. |
How are the billions of nerve axons in the body formed? What directs their growth? Biologists were intrigued with these questions, which seemed to have no easy solutions. Since a single nerve axon may be more than a meter in length (for example, motor nerves running from the spinal cord to the toes), it seemed impossible that a single cell could reach out so far. One hypothesis was that numerous neural cells joined together in a chain to form an axon. It was alternatively suggested that an axon grew from a series of preformed protoplasmic bridges along its route. The answer had to await the development of one of the most powerful tools available to biologists, the cell culture technique.
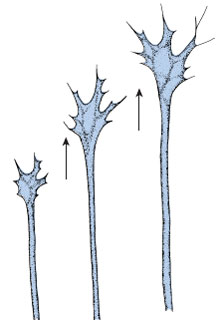 |
| Figure 8-26 Growth cone at the growing tip of a nerve axon. Materials for growth flow down the axon to the growth cone from which numerous threadlike filopodia extend. These serve as a pioneering guidance system for the developing axon. Direction of growth is shown by arrows. |
The second question—what directs nerve growth—has taken longer to unravel. An idea held well into the 1940s was that nerve growth is a random, diffuse process. A major hypothesis proposed that the nervous system developed as an equipotential network, or blank slate, that later would be shaped by usage into a functional system. The nervous system just seemed too incredibly complex for us to imagine that nerve fibers could find their way selectively to so many predetermined destinations. Yet it appears that this is exactly what they do! Research with invertebrate nervous systems indicated that each of the billions of nerve cell axons acquires a distinct identity that in some way directs it along a specific pathway to its destination. Many years ago Harrison observed that a growing nerve axon terminated in a growth cone, from which extend numerous tiny threadlike pseudopo-dial processes (filopodia) (Figure 8-26). Recent research has shown that the growth cone is steered by an array of guidance molecules secreted along the pathway and by the axon’s target. This chemical guidance system, which must, of course, be genetically directed, is just one example of the amazing precision that characterizes the entire process of differentiation.
The tissue culture technique developed by Ross G. Harrison is now used extensively by scientists in all fields of active biomedical research, not just by developmental biologists. The great impact of the technique has been felt only in recent years. Harrison was twice considered for the Nobel Prize (1917 and 1933), but he failed ever to receive the award because, ironically, the tissue culture method was then believed to be “of rather limited value.”




