Alkaloids Derived from Ornithine
L-Ornithine (Figure 1) is a non-protein amino acid forming part of the urea cycle in animals, where it is produced from L-arginine in a reaction catalysed by the enzyme arginase. In plants it is formed mainly from L-glutamate (Figure 2). Ornithine contains both δ- and α-amino groups, and it is the nitrogen from the former group which is incorporated into alkaloid structures along with the carbon chain, except for the carboxyl group.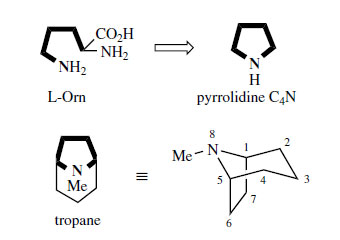 |
| Figure 1 |
Thus ornithine supplies a C4N building block to the alkaloid, principally as a pyrrolidine ring system, but also as part of the tropane alkaloids (Figure 1). Most of the other amino acid alkaloid precursors typically supply nitrogen from their solitary α-amino group. However, the reactions of ornithine are almost exactly paralleled by those of L-lysine, which incorporates a C5N unit containing its ε-amino group (see Alkaloids Derived from Lysine).




