Terpenoid Indole Alkaloids
More than 3000 terpenoid indole alkaloids are recognized,
making this one of the major groups
of alkaloids in plants. They are found mainly in eight plant families, of which the Apocynaceae,
the Loganiaceae, and the Rubiaceae provide the
best sources. In terms of structural complexity,
many of these alkaloids are quite outstanding, and
it is a tribute to the painstaking experimental studies
of various groups of workers that we are able
biochemical origins. In virtually all structures, a
tryptamine portion can be recognized. The remaining
fragment is usually a C9 or C10 residue, and
three main structural types are discernable. These
are termed the Corynanthe type, as in ajmalicine and akuammicine, the Aspidosperma type, as in tabersonine, and the Iboga type, exemplified by catharanthine (Figure 75).The C9 or C10 fragment
was shown to be of terpenoid origin, and
the secoiridoid secologanin was
identified as the terpenoid derivative, which initially
combined with the tryptamine portion of
the molecule. |
Furthermore, the Corynanthe, Aspidosperma, and Iboga groups of alkaloids could be related and rationalized in terms of rearrangements occurring in the terpenoid part of the structures (Figure 75). Secologanin itself contains the ten-carbon framework typical of the Corynanthegroup. The Aspidosperma and Iboga groups could then arise by rearrangement of the Corynanthe skeleton as shown. This is represented by detachment of a three-carbon unit, which is then rejoined to the remaining C7 fragment in one of two different ways. Where C9 terpenoid units are observed, the alkaloids normally appear to have lost the carbon atom indicated in the circle. This corresponds to the carboxylate function of secologanin and its loss by hydrolysis/decarboxylation is now understandable.
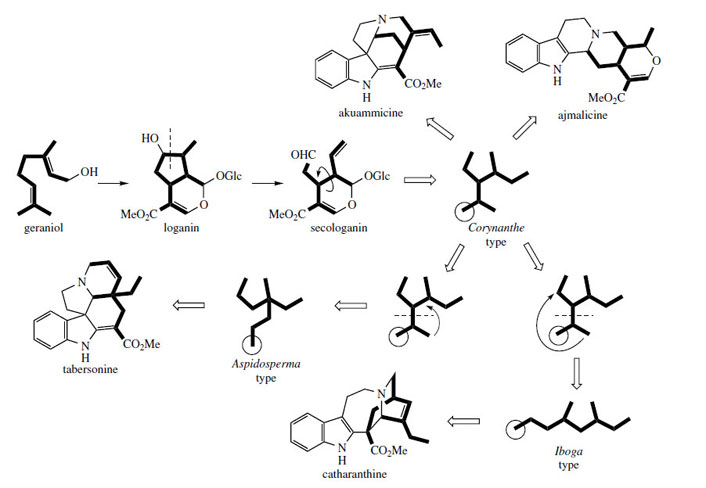 |
| Figure 75 |
Condensation of secologanin with tryptamine in a Mannich-like reaction generates the tetrahydro- β-carboline system and produces strictosidine(Figure 76). Hydrolysis of the glycoside function allows opening of the hemiacetal, and exposure of an aldehyde group, which can react with the secondary amine function giving a quaternary Schiff base. These reactions are also seen in the pathway to ipecac alkaloids. Allylic isomerization, moving the vinyl double bond into conjugation with the iminium generates dehydrogeissoschizine, and cyclization to cathenamine follows. Cathenamine is reduced to ajmalicine in the presence of NADPH.
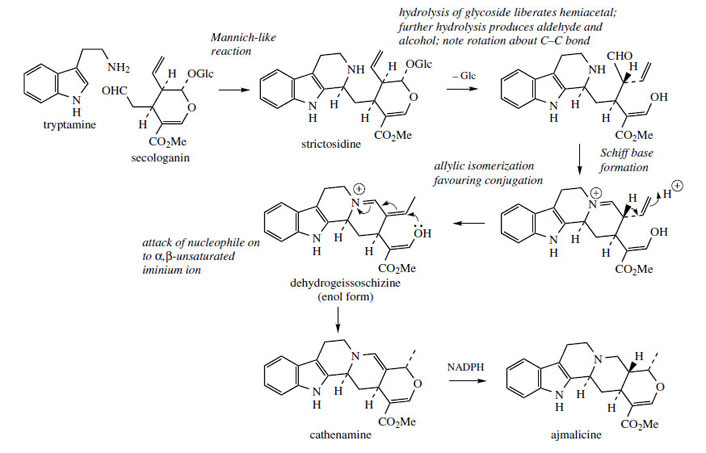 |
| Figure 76 |
Carbocyclic variants related to ajmalicine such as yohimbine are likely to arise from dehydrogeissoschizine by the mechanism indicated in Figure 77. Yohimbine is found in Yohimbe bark (Pausinystalia yohimbe; Rubiaceae) and Aspidosperma bark (Aspidosperma species; Apocynaceae) and has been used in folk medicine as an aphrodisiac. It does have some pharmacological activity and is known to dilate blood vessels. More important examples containing the same carbocyclic ring system are the alkaloids found in species of Rauwolfia*, especially R. serpentina (Apocynaceae). Reserpine and deserpidine(Figure 78) are trimethoxybenzoyl esters of yohimbine-like alkaloids, whilst rescinnamine is a trimethoxycinnamoyl ester. Both reserpine and rescinnamine contain an additional methoxyl substituent on the indole system at position 11, the result of hydroxylation and methylation at a late stage in the pathway. A feature of these alkaloids is that they have the opposite stereochemistry at position 3 to yohimbine and strictosidine. Rauwolfia serpentina also contains significant amounts of ajmalicine (Figure 76), emphasizing the structural and biosynthetic relationships between the two types of alkaloid.
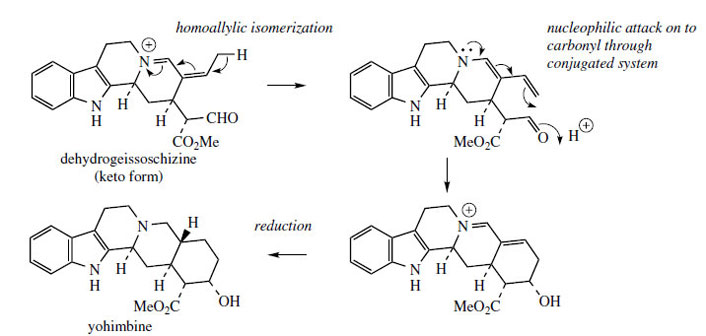 |
| Figure 77 |
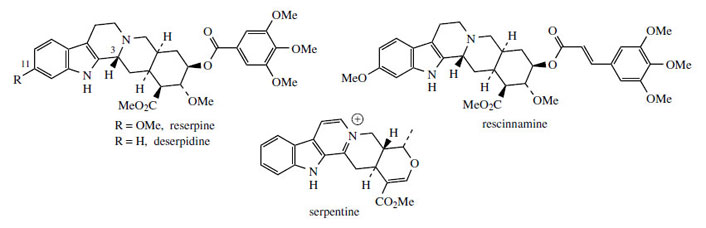 |
| Figure 78 |
Rauwolfia
Rauwolfia has been used in Africa for hundreds of years, and in India for at least 3000 years. It was used as an antidote to snake-bite, to remove white spots in the eyes, against stomach pains, fever, vomiting, and headache, and to treat insanity. It appeared to be a universal panacea, and was not considered seriously by Western scientists until the late 1940s/early 1950s. Clinical tests showed the drug to have excellent antihypertensive and sedative activity. It was then rapidly and extensively employed in treating high blood pressure and to help mental conditions, relieving anxiety and restlessness, and thus initiating the tranquillizer era. The 'cure for insanity' was thus partially justified, and rauwolfia was instrumental in showing that mental disturbance has a chemical basis and may be helped by the administration of drugs.
Rauwolfia is the dried rhizome and roots of Rauwolfia (sometimes Rauvolfia) serpentina (Apocynaceae) or snakeroot, a small shrub from India, Pakistan, Burma, and Thailand. Other species used in commerce include R. vomitoria from tropical Africa, a small tree whose leaves after ingestion cause violent vomiting, and R. canescens (= R. tetraphylla) from India and the Caribbean. Most of the drug material has been collected from the wild. Rauwolfia serpentinacontains a wide range of indole alkaloids, totalling 0.7-2.4%, though only 0.15-0.2% consists of desirable therapeutically active compounds, principally reserpine, rescinnamine, and deserpidine (Figure 78). Other alkaloids of note are serpentine (Figure 78), ajmalicine (Figure 76), and ajmaline (Figure 82). Reserpine and deserpidine are major alkaloids in R. canescens, and R. vomitoria contains large amounts of rescinnamine and reserpine.
Reserpine and deserpidine (Figure 78) have been widely used as antihypertensives and mild tranquillizers. They act by interfering with catecholamine storage, depleting levels of available neurotransmitters. Prolonged use of the pure alkaloids, reserpine in particular, has been shown to lead to severe depression in some patients, a feature not so prevalent when the powdered root was employed. The complex nature of the alkaloidal mixture means the medicinal action is somewhat different from that of reserpine alone. Accordingly, crude powdered rauwolfia remained an important drug for many years, and selected alkaloid fractions from the crude extract have also been widely used. The alkaloids can be fractionated according to basicity. Thus, serpentine and similar structures are strongly basic, whilst reserpine, rescinnamine, deserpidine and ajmalicine are weak bases. Ajmaline and related compounds have intermediate basicity.
The rauwolfia alkaloids are now hardly ever prescribed in the UK, either as antihypertensives or as tranquillizers. Over a period of a few years, they have been rapidly superseded by synthetic alternatives. Reserpine has also been suggested to play a role in the promotion of breast cancers. Both ajmalicine (= raubasine) (Figure 76) and ajmaline (Figure 82) are used clinically in Europe, though not in the UK. Ajmalicine is employed as an antihypertensive, whilst ajmaline is of value in the treatment of cardiac arrhythmias. Ajmalicine is also extracted commercially from Catharanthus roseus.
The structural changes involved in converting the Corynanthe type skeleton into those of the Aspidospermaand Iboga groups are quite complex, and are summarized in Figure 79. Early intermediates are alkaloids such as preakuammicine, which, although clearly of the Corynanthe type, is sometimes designated as Strychnos type (compare strychnine, page 358). This is because the Corynantheterpenoid unit, originally attached to the indole α-carbon, is now bonded to the β-carbon, and a new bonding between the rearrangeable C3 unit and C-α is in place. Stemmadenine arises through fission of the bond to C-β, and then further fission yields a hypothetical intermediate, the importance of which is that the rearrangeable C3 unit has been cleaved from the rest of the terpenoid carbons. Alkaloids of the Aspidosperma type, e.g. tabersonine and vindoline, and Iboga type, e.g. catharanthine, then arise from this intermediate by different bonding modes (Figure 79).
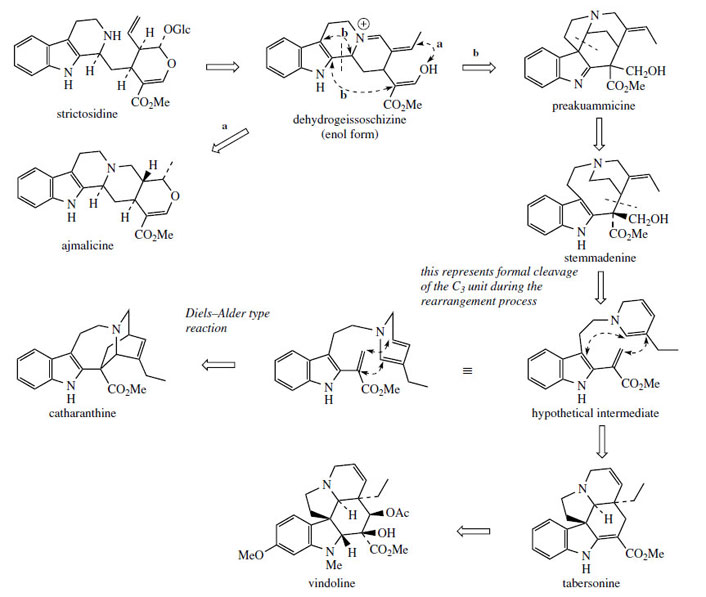 |
| Figure 79 |
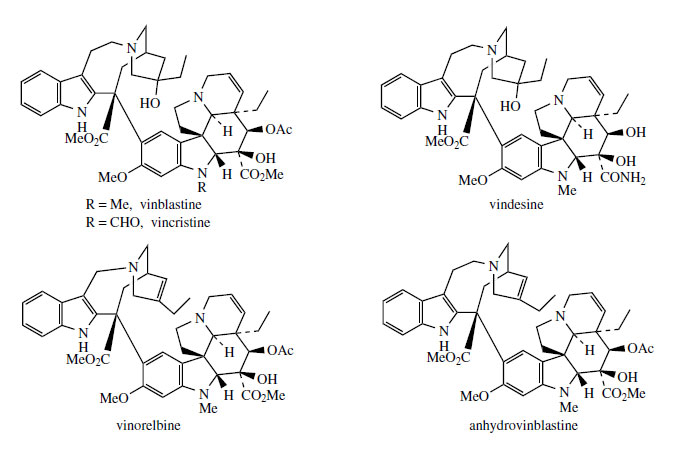
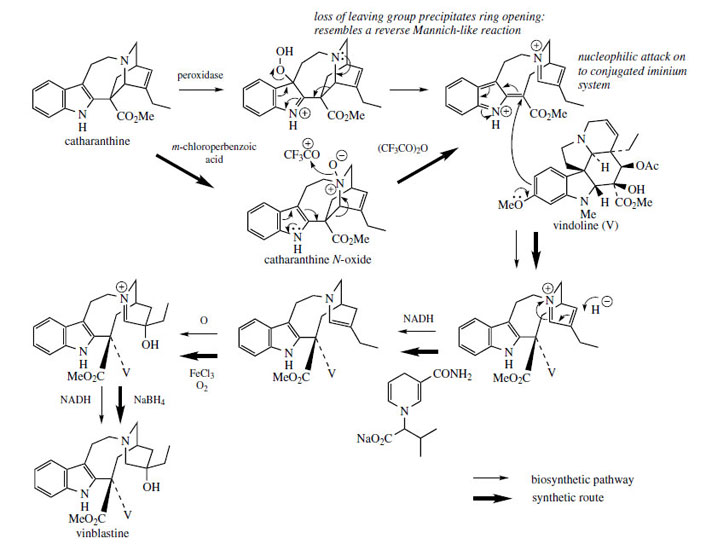
Many of the experimental studies that have led to an understanding of terpenoid indole alkaloid biosynthesis have been carried out using plants of the Madagascar periwinkle (Catharanthus roseus*, formerly Vinca rosea; Apocynaceae). Representatives of all the main classes of these alkaloids are produced, including ajmalicine (Corynanthe), catharanthine (Iboga), and vindoline (Aspidosperma). The sequence of alkaloid formation has been established initially by noting which alkaloids become labelled as a feeding experiment progresses, and more recently by appropriate enzymic studies. However, the extensive investigations of the Catharanthus roseus alkaloids have also been prompted by the anticancer activity detected in a group of bisindole alkaloids. Two of these, vinblastine and vincristine (Figure 80), have been introduced into cancer chemotherapy and feature as some of the most effective anticancer agents available. These structures are seen to contain the elements of catharanthine and vindoline, and, indeed, they are derived by coupling of these two alkaloids. The pathway is believed to involve firstly an oxidative reaction on catharanthine, catalysed by a peroxidase, generating a, peroxide which loses the peroxide as a leaving group, breaking a carbon–carbon bond as shown (Figure 81). This intermediate electrophilic ion is attacked by the nucleophilic vindoline, C-5 of the indole nucleus being suitably activated by the OMe at C-6 and also by the indole nitrogen. The adduct is then reduced in the dihydropyridinium ring by NADH-dependent 1,4-addition, giving the substrate for hydroxylation. Finally, reduction yields vinblastine. Vincristine, with its N-formyl group rather than N-methyl on the vindoline fragment, may be an oxidized product from vinblastine.
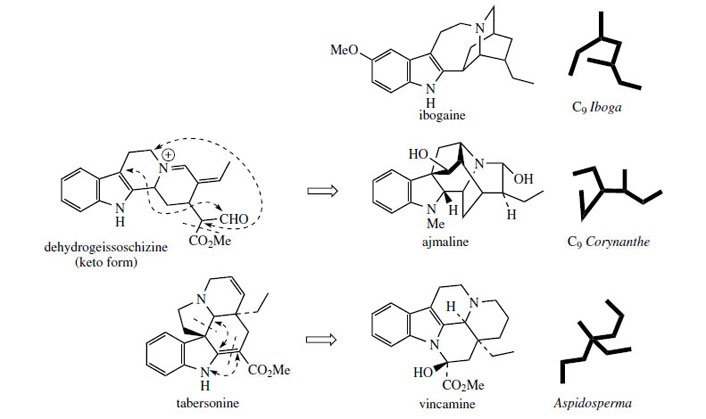
Further variants on the terpenoid indole alkaloid skeleton (Figure 82) are found in ibogaine from Tabernanthe iboga*, vincamine from Vinca minor, and ajmaline from Rauwolfia serpentina. Ibogaine is simply a C9 Iboga type alkaloid, but is of interest as an experimental drug to treat heroin addiction. In a number of European countries, vincamine is used clinically as a vasodilator to increase cerebral blood flow in cases of senility, and ajmaline for cardiac arrhythmias. Ajmaline contains a C9 Corynanthe type unit and its relationship to dehydrogeissoschizine is indicated in Figure 82. Vincamine still retains a C10 Aspidospermaunit, and it originates from tabersonineby a series of reactions that involve cleavage of bonds to both α and β positions of the indole
(Figure 82).
 |
| Figure 80 |
 |
| Figure 81 |
Catharanthus
The Madagascar periwinkle Catharanthus roseus (= Vinca rosea) (Apocynaceae) is a small herb or shrub originating in Madagascar, but now common in the tropics and widely cultivated as an ornamental for its shiny dark green leaves and pleasant five-lobed flowers. Drug material is now cultivated in many parts of the world, including the USA, Europe, India, Australia, and South America.
Because of its folklore usage as a tea for diabetics, the plant was originally investigated for potential hypoglycaemic activity. Although plant extracts had no effects on blood sugar levels in rabbits, the test animals succumbed to bacterial infection due to depleted white blood cell levels (leukopenia). The selective action suggested anticancer potential for the plant, and an exhaustive study of the constituents was initiated. The activity was found in the alkaloid fraction, and more than 150 alkaloids have been characterized in the plant. These are principally terpenoid indole alkaloids, many of which are known in other plants, especially from the same family. Useful antitumour activity was demonstrated in a number of dimeric indole alkaloid structures (more correctly bis-indole alkaloids), including vincaleukoblastine, leurosine, leurosidine, and leurocristine. These compounds became known as vinblastine, vinleurosine, vinrosidine, and vincristine respectively, the vin- prefix being a consequence of the earlier botanical nomenclature Vinca rosea, which was commonly used at that time. The alkaloids vinblastine and vincristine (Figure 80) were introduced into cancer chemotherapy and have proved to be extremely valuable drugs.
Despite the minor difference in structure between vinblastine and vincristine, a significant difference exists in the spectrum of human cancers that respond to the drugs. Vinblastine(Figure 80) is used mainly in the treatment of Hodgkin's disease, a cancer affecting the lymph glands, spleen, and liver. Vincristine (Figure 80) has superior antitumour activity compared to vinblastine but is more neurotoxic. It is clinically more important than vinblastine, and is especially useful in the treatment of childhood leukaemia, giving a high rate of remission. Some other cancer conditions, including lymphomas, small cell lung cancer, and cervical and breast cancers, also respond favourably. The alkaloids need to be injected, and both generally form part of a combination regimen with other anticancer drugs. Vindesine (Figure 80) is a semi-synthetic derivative of vinblastine, which has been introduced for the treatment of acute lymphoid leukaemia in children. Vinorelbine (Figure 80), an anhydro derivative of 8´-norvinblastine, is a newer semi-synthetic modification obtained from anhydrovinblastine (Figure 80), where the indole.C2N bridge in the catharanthine-derived unit has been shortened by one carbon. It is orally active and has a broader anticancer activity yet with lower neurotoxic side-effects than either vinblastine or vincristine. These compounds all inhibit cell mitosis, acting by binding to the protein tubulin in the mitotic spindle, preventing polymerization into microtubules, a mode of action shared with other natural agents, e.g. colchicine and podophyllotoxin.
A major problem associated with the clinical use of vinblastine and vincristine is that only very small amounts of these desirable alkaloids are present in the plant. Although the total alkaloid content of the leaf can reach 1% or more, over 500 kg of catharanthus is needed to yield 1 g of vincristine. This yield (0.0002%) is the lowest of any medicinally important alkaloid isolated on a commercial basis. Extraction is both costly and tedious, requiring large quantities of raw material and extensive use of chromatographic fractionations. The growing importance of vincristine relative to vinblastine as drugs is not reflected in the plant, which produces a much higher proportion of vinblastine. Fortunately, it is possible to convert vinblastine into vincristine by controlled chromic acid oxidation or via a microbiological N-demethylation using Streptomyces albogriseolus. Considerable effort has been expended on the semisynthesis of the 'dimeric' alkaloids from 'monomers' such as catharanthine and vindoline, which are produced in C. roseus in much larger amounts. Efficient, stereospecific coupling has eventually been achieved, and it is now possible to convert catharanthine and vindoline into vinblastine in about 40% yield. The process used is a biomimetic one, virtually identical to the suggested biosynthetic process, and is also included in Figure 81. Catharanthine- N-oxide is employed instead of the peroxidase-generated peroxide, and this couples readily in trifluoroacetic anhydride with vindoline in almost quantitative yield. Subsequent reduction, oxidation, and reduction steps then give vinblastine via the same 'biosynthetic' intermediates. It is particularly interesting that the most effective reducing agents for the transformation of the dihydropyridinium compound into the tetrahydropyridine were N-substituted 1,4- dihydronicotinamides, simpler analogues of NADH, the natural reducing agent. Excellent yields of anhydrovinblastine (the starting material for vinorelbine production) (Figure 80) can also be obtained by electrochemical oxidation of catharanthine/vindoline. These syntheses should improve the supply of these alkaloids and derivatives, and also allow more detailed studies of structure-activity relationships to be undertaken. This group of compounds is still of very high interest, and development programmes for analogues continue.
Ajmalicine is present in the roots of Catharanthus roseus at a level of about 0.4%, and this plant is used as a commercial source in addition to Rauwolfia serpentina.
Iboga
The Iboga group of terpenoid indole alkaloids takes its name from Tabernanthe iboga (Apocynaceae), a shrub from the Congo and other parts of equatorial Africa. Extracts from the root bark of this plant have long been used by indigenous people in rituals, to combat fatigue, and as an aphrodisiac. The root bark contains up to 6% indole alkaloids, the principal component of which is ibogaine (Figure 82). Ibogaine is a CNS stimulant, and is also psychoactive. In large doses, it can cause paralysis and respiratory arrest. Ibogaine is of interest as a potential drug for relieving heroin craving in drug addicts. Those who use the drug experience hallucinations from the ibogaine, but it is claimed they emerge from this state with a significantly reduced opiate craving. A number of deaths resulting from the unsupervised use of ibogaine has led to its being banned in some countries.
Alkaloids like preakuammicine (Figure 79) and akuammicine (Figure 75) contain the C10 and C9 Corynanthe type terpenoid units respectively. They are, however, representatives of a subgroup of Corynanthe alkaloids termed the Strychnos type because of their structural similarity to many of the alkaloids found in Strychnos species (Loganiaceae), e.g. S. nux-vomica*, noteworthy examples being the extremely poisonous strychnine (Figure 83) and its dimethoxy analogue brucine (Figure 84). The non-tryptamine portion of these compounds contains 11 carbons, and is constructed from an iridoid-derived C9 unit, plus two further carbons supplied from acetate. The pathway to strychnine in Figure 83 involves loss of one carbon from a preakuammicine-like structure via hydrolysis/decarboxylation and then addition of the extra two carbons by aldol condensation with the formyl group, complexed as a hemiacetal in the so-called Wieland–Gumlich aldehyde. The subsequent formation of strychnine from this hemiacetal is merely construction of ether and amide linkages.
 |
| Figure 82 |
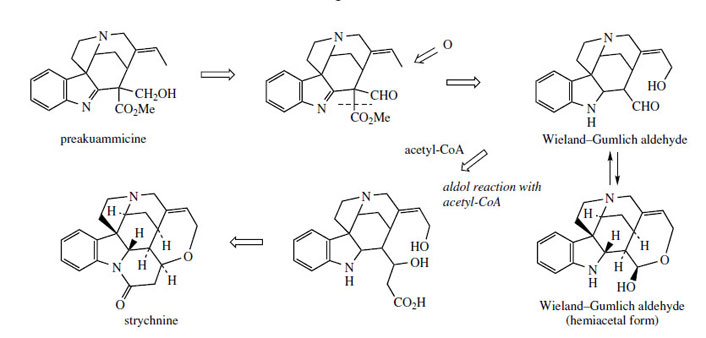 |
| Figure 83 |
Nux-vomica
Nux-vomica consists of the dried ripe seeds of Strychnos nux-vomica (Loganiaceae), a small tree found in a wide area of East Asia extending from India to Northern Australia. The fruit is a large berry with a hard coat and a pulpy interior containing three to five flattish grey seeds. These seeds contain 1.5-5% of alkaloids, chiefly strychnine (about 1.2%) and brucine (about 1.6%) (Figure 82). Strychnine is very toxic, affecting the CNS and causing convulsions. This is a result of binding to receptor sites in the spinal cord that normally accommodate glycine. Fatal poisoning (consumption of about 100 mg by an adult) would lead to asphyxia following contraction of the diaphragm. It has found use as a vermin killer, especially for moles. Its only medicinal use is in very small doses as an appetite stimulant and general tonic, sometimes with iron salts if the patient is anaemic. Brucine is considerably less toxic. Both compounds have been regularly used in synthetic chemistry as optically active bases to achieve optical resolution of racemic acids. Seeds of the related Strychnos ignatii have also served as a commercial source of strychnine and brucine.
Of biochemical interest is the presence of quite significant amounts (up to 5%) of the iridoid glycoside loganin in the fruit pulp of Strychnos nux-vomica. This compound is, of course, an intermediate in the biosynthesis of strychnine and other terpenoid indole alkaloids.
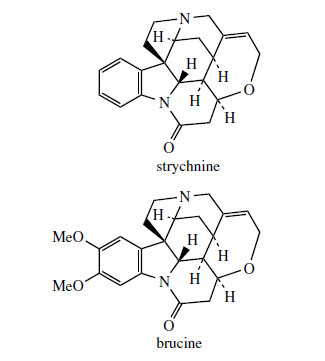 |
| Figure 84 |
The arrow poison curare, when produced from Chondrodendron species (Menispermaceae), contains principally the bis-benzyltetrahydroisoquinoline alkaloid tubocurarine. Species of Strychnos, especially S. toxifera, are employed in making loganiaceous curare, and biologically active alkaloids isolated from such preparations have been identified as a series of toxiferines, e.g. C-toxiferine (Figure 85). The structures appear remarkably complex, but may be envisaged as a combination of two Wieland–Gumlich aldehyde-like molecules (Figure 85). The presence of two quaternary nitrogens, separated by an appropriate distance, is responsible for the curare-like activity (compare tubocurarine and synthetic analogues, page 326). Alcuronium(Figure 85) is a semi-synthetic skeletal muscle relaxant produced from C-toxiferine.
Ellipticine* (Figure 86) contains a pyridocarbazole skeleton, which is also likely to be formed from a tryptamine–terpenoid precursor. Although little evidence is available, it is suggested that a precursor like stemmadenine may undergo transformations that effectively remove the two-carbon bridge originally linking the indole and the nitrogen in tryptamine (Figure 86). The remaining C9 terpenoid fragment now containing the tryptamine nitrogen can then be used to generate the rest of the skeleton. Ellipticine is found in Ochrosia elliptica (Apocynaceae) and related species and has useful anticancer properties.




