Preparation of Cilia from Human Airway Epithelial Cells
For many years, the biochemical analysis of cilia, and flagella has focused primarily on organisms that are easy to grow and maintain in the laboratory and from which axonemal structures can be isolated in large quantities with a high degree of purity. For example, the isolation of flagella from Chlamydomonas in response to pH shock was described as early as 1972 (Witman et al., 1972) and cilia were isolated from Tetrahymena using dibucaine in 1974 (Thompson et al., 1974). While studies of these and other organisms have provided a wealth of valuable information concerning the structure and function of these fascinating axonemal structures, it is clearly important to also investigate the structure, function, and regulation of mammalian, especially human, cilia and flagella. While the axoneme of mammalian sperm has been isolated by a number of techniques (e.g., San Agustin and Witman, 1995), only a few reports detail isolations of mammalian cilia from the airway. This probably reflects in part the difficulty of obtaining sufficient quantities of suitable starting material and the inherent difficulties encountered when working with whole tissues.
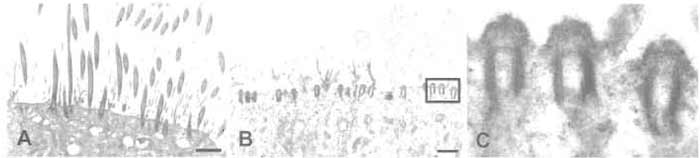 |
| FIGURE 1 Electron micrographs of ciliated cultures of human airway epithelial cells before (A) and after (B and C) isolation of cilia. (A) A heavily ciliated culture after 6 weeks of culture. (B) A parallel culture fixed immediately after removal of cilia. Note the intact basal bodies, which are shown at higher magnification in C. Scale bar in A and B: 2.5 µm. |
Protease inhibitor cocktail (P8340), dithiothreitol (DTT, D-9779), and Triton X-100 (T-8787) are from Sigma. β-Mercaptoethanol (0482) is from Amresco. The protease inhibitor cocktail is thawed upon arrival and frozen in small aliquots (100-200 µl). A 10% solution of Triton X-100 is made in sterile distilled water and stored in the refrigerator for no longer than 1 month. DTT is conveniently frozen in small aliquots at 100mM. All other chemicals are standard laboratory reagents and can be obtained from most laboratory suppliers.
- Deciliation buffer (DB): 10mM Tris-HCl (pH 7.5), 50 mM NaCl, 10 mM CaCl2, 1 mM EDTA, 0.1% Triton X-100, 7mM β-mercaptoethanol, and 1% protease inhibitor cocktail. Prepare a stock solution without protease inhibitor, β-mercaptoethanol, or Triton X-100. Sterile filter and store in the refrigerator. Before starting the procedure, add to 4.9ml of the stock solution 50µl of freshly thawed protease inhibitor cocktail, 50 µl of 10% Triton X-100, and 2.45 µl of β-mercaptoethanol.
- Resuspension buffer (RB): 30mM HEPES (pH 7.3), 25mM NaCl, 5mM MgSO4, 1 mM EGTA, 0.1mM EDTA, 1 mM DTT, and 1% protease inhibitor cocktail. Prepare a 10× stock solution without protease inhibitor and DTT (300mM HEPES, 250mM NaCl, 50mM MgSO4,10mM EGTA, 1 mM EDTA), sterile filter, and store in the refrigerator. Before starting the isolation procedure, dilute an aliquot to 1X with sterile water. To 4.9ml of diluted stock, add 50µl of freshly thawed protease inhibitor cocktail and 50µl of freshly thawed 100 mM DTT.
As noted earlier, this procedure starts with heavily ciliated cultures of human airway epithelial cells. Cilia can be isolated from four 30-mm Millicell-CM culture inserts (PICM03050; Millipore) at one time. The inserts are first transferred to a six-well plate for ease of manipulation. Once started, the procedure should be carried out quickly.
- Gently wash the mucus from the apical surface of the culture with at least two rinses of 2 ml of chilled phosphate-buffered saline (PBS). Add the PBS to the surface of the culture, swirl gently, and aspirate. If the cultures are producing large amounts of mucus, perform additional washes until the visible mucus is removed. After the last wash, carefully aspirate as much fluid as possible from both apical and basolateral sides of the insert.
- Add 300µl of cold DB to each insert.
- Cover the six-well plate and rock vigorously for 1 min. Tilt the plate from side to side so the solution flows back and forth. Occasionally swirling the plate so the solution washes around the edges helps remove cilia from this area of the insert. Tilt the plate slightly so that the solution collects on one edge and remove with a pipetter. Pool the solution containing the cilia from two inserts into one tube. Keep isolated cilia on ice.
- Repeat steps 2 and 3. Pool the second collection of cilia with the first. There should be 1.2 ml per microcentrifuge tube.
- Pellet any cellular debris by centrifuging at 1000 RCF for 1 min at 4°C. Carry out the remaining steps in a cold room.
- Collect the cilia containing supernatant into a new tube. When starting with heavily ciliated cultures that are washed well, there will be only a very small pellet of debris, with some stringy material along the sides of the tube. Try to remove the supernatant without disturbing the pellet. We have found that spinning at higher speeds or for longer times pellets cilia as well as debris. Depending on the goal of the experiment (high purity or greatest recovery), the time of this centrifugation may be adjusted.
- Pellet the ciliary axonemes by centrifugation at 12,000 RCF for 5 min at 4°C. After this centrifugation, a compact white pellet should be obtained. Carefully remove the supernatant and discard.
- Resuspend the ciliary axonemes in 0.5 ml of RB buffer/insert (1.0ml per tube). The pellet does not go into solution readily and must be pipetted gently to disrupt. While a homogeneous solution is desired, small clumps of material do not seem to affect the results.
- Optional: To remove ciliary membranes, add 50 µl of 10% Triton-X 100 (final concentration of 0.5%) and incubate the solution on ice for 15min. Repeat steps 7 and 8.
- Withdraw and spot 5µl of the solution onto a slide, coverslip, and examine by phase-contrast microscopy at 40× magnification. The axonemes should be readily visible as individual strands, with little cellular debris present (Fig. 2A).
- If a protein determination is desired, remove an aliquot to a separate tube (typically 10%, or 100µl). Pellet the cilia in both the sample and the protein determination tube by centrifugation at 12,000 RCF for 5 min at 4°C.
- Remove as much of the supernatant as possible and freeze the pellets at -80°C until needed.
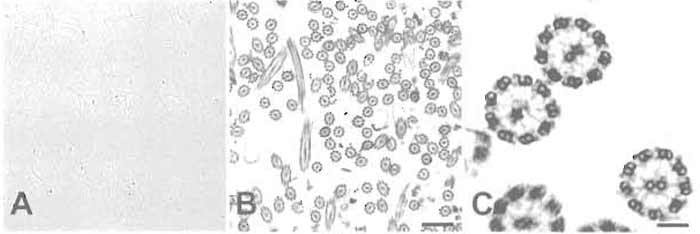 |
| FIGURE 2 (A) Phase-contrast light micrograph of isolated cilia showing the appearance of the preparation after step 10 (original magnification, 400×). (B) Electron micrograph showing many intact ciliary axonemes after step 12. (C) Higher magnification of the same preparation as in B showing details of the isolated axonemes. Note the presence of radial spokes and dynein arms. Scale bars: 1.1 µm (B) and 0.15µm (C). |
- Protein concentration is determined using the BCA protein assay kit (Pierce, Rockford, IL). To solublize the axonemes, the pellet is dissolved in 0.5% SDS before performing the assay. The protein values obtained provide estimates of how much protein is in the cilia pellet. Although the amount of ciliary axonemes recovered varies with the degree of ciliated cell differentiation, each insert typically yields about 50 µg of protein.
- The microscopic examination of the preparation in step 10 provides a simple and rapid qualitative estimate of the purity of the axonemes recovered.
- Cilia isolated by this procedure can be reactivated by the addition of ATP to the cilia suspension obtained after step 10. ATP is added to an aliquot of the cilia and the reactivation is observed by phasecontrast microscopy (Hastie et al., 1986).
- For detailed analysis, the pelleted axonemes can be fixed in 2% formaldehyde/2% glutaraldehyde with 0.5% tannic acid (Hayat, 1989) and examined by transmission electron microscopy. An example of a routine preparation of isolated axonemes examined by electron microscopy is shown in Figs. 2B and 2C.
- A problem sometimes encountered has been the fragmentation of the axoneme into individual microtubules. While the cause of this phenomena has not been identified, proteolytic degradation or the use of old or improperly made solutions seems to be a likely cause. When this is observed, fresh solutions should be prepared and the procedure repeated to verify that intact axonemes are obtained.
This work was funded in part by NIH Grant HL63103 from the National Heart, Lung, and Blood Institute. The author thanks Dr. Scott Randell and the members of the Cell and Tissue Core Facility for helpful discussions and providing the human airway epithelial cells, Kim Burns and the members of the Histology Core Facility for providing electron microscopy services, Kerri Kendrick for preparing the illustrations, and Dr. William Reed for helpful suggestions concerning the cilia isolation protocol.
References
Witman, G. B., Carlson, K., Berliner, J., and Rosenbaum, J. L. (1972). Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes, J. Cell Biol. 54, 507-539.
Thompson, G. A., Jr., Baugh, L. C., and Walker, L. E (1974). Nonlethal deciliation of Tetrahymena by a local anesthetic and its utility as a tool for studying cilia regeneration. J. Cell Biol. 61, 253-257.
San Agustin, J. T., and Witman, G. B. (1995). Isolation of ram sperm flagella. Methods Cell Biol. 47, 31-36.
Zhang, Y. J., O'Neal, W. K., Randell, S. H., Blackburn, K., Moyer, M. B., Boucher, R. C., and Ostrowski, L. E. (2002). Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia, J. Biol Chem. 277, 17906-17915.
Reed, W., Carson, J. L., Moats-Staats, B. M., Lucier, T., Hu, P., Brighton, L., Gambling, T. M., Huang, C. H., Leigh, M. W., and Collier, A. M. (2000). Characterization of an axonemal dynein heavy chain expressed early in airway epithelial ciliogenesis. Am. J. Respir. Cell Mol. Biol. 23, 734-741.
Ostrowski, L. E., Blackburn, K., Radde, K. M., Moyer, M. B., Schlatzer, D. M., Moseley, A., and Boucher, R. C. (2002). A proteomic analysis of human cilia: Identification of novel components. Mol. Cell Prot. 1, 451-465.
Kultgen, P. L., Byrd, S. K., Ostrowski, L. E., and Milgram, S. L. (2002). Characterization of an a-kinase anchoring protein in human ciliary axonemes. Mol. Biol. Cell. 13, 4156-4166.
Salathe, M., Pratt, M. M., and Wanner, A. (1993). Cyclic AMPdependent phosphorylation of a 26 kD axonemal protein in ovine cilia isolated from small tissue pieces. Am. J. Respir. Cell Mol. Biol. 9, 306-314.
Bernacki, S. H., Nelson, A. L., Abdullah, L., Sheehan, J. K., Harris, A., William Davis, C., and Randell, S. H. (1999). Mucin gene expression during differentiation of human airway epithelia in vitro: Muc4 and muc5b are strongly induced. Am. J. Respir. Cell Mol. Biol. 20, 595-604.
Randell, S. H., Walstad, L., Schwab, U. E., Grubb, B. R., and Yankaskas, J. R. (2001). Isolation and culture of airway epithelial cells from chronically infected human lungs. in vitro Cell Dev. Biol. Anim. 37, 480-489.
Hayat, M. (1989). "Principles and Techniques of Electron Microscopy," 3rd Edn. CRC Press, Boca Raton, FL.




