Flame tests
Simple flame tests can be carried out on solid samples. Place a little of the solid on a watch-glass and moisten with a drop of concentrated hydrochloric acid. The purpose of the hydrochloric acid is to produce metal chlorides which are volatile at the temperature of the Bunsen burner.Pre-clean a platinum or nichrome wire by holding it in the hottest part of the Bunsen flame (just above the central blue cone) until there is no coloured flame from the wire. Cool, then dip the cleaned wire into the moistened solid sample. Place the wire at the edge of the Bunsen flame (Fig. 19.3) and record the colour of the flame from the sample (see Table 19.2).
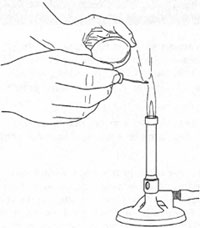 |
| Fig. 19.3 Holding a nichrome wire in a flame test. |
 |
| Table 19.2 Flame tests for cations |
** Viewing through cobalt-blue glass also allows calcium and strontium to be distinguished. In this case, calcium is light green in colour while strontium appears purple.




