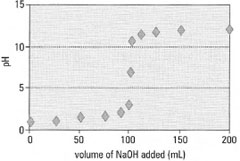
Neutralization curves
| A plot of pH against the volume of alkali added (mL) is known as a
neutralization or titration curve (Fig. 22.2). The curve is generated by a
'potentiometric titration' in which pH is measured after each addition of
alkali (or acid). The significant feature of the curve is the very sharp and
sudden change in pH near to the equivalence point of the titration. For a
strong acid and alkali this will occur at pH 7. If either the acid or base
concentration is unknown, a preliminary titration is necessary to find the
approximate equivalence point followed by a more accurate titration. The ideal pH range for an indicator is 4.5-9.5. Determination of the equivalence point From the neutralization curve (Fig. 22.2), the initial and final slopes are drawn (Fig. 22.3) and a parallel line is drawn such that the mid-point is on the curve. This is the equivalence point, producing a titration value of xmL. |
|
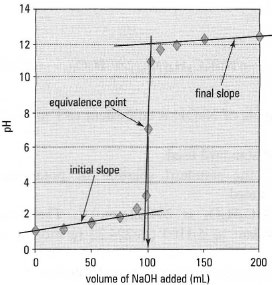 |
| Figure 22.3 Determination of the equivalence point. |
Example calculations
Standardization of a sodium hydroxide solution
What is the molarity of a solution of sodium hydroxide, 25.0 mL of which requires 21.0 mL of a standard solution of hydrochloric acid of concentration 0.100 mol L−1 for neutralization? Following the sequence in Box 21.1:
- Write the equation:
⇒Equation [22.2]
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(I) - Determine the equivalences. Equation [22.2] shows that 1 mole of NaOH requires 1 mole of HCI for neutralization, i.e. 1 mole of NaOH is equivalent to 1 mole of HCI.
- Calculate the number of moles of standard used.
Number of moles of HCL = concentration (mol L−1) × volume (mL) 1000
= 0.100 mol L−1 × 21.0mL 1000
= 2.1 × 10−3mol
- Calculate the number of moles of NaOH in the initial volume of test
solution.
As indicated in point 2 above, 1 mole of NaOH is equivalent to 1 mole of HCI. Therefore:
no. of moles of NaOH in initial volume = no. of moles of HCl used
= 2.1 × 10−3mol - Determine the concentration of the NaOH solution.
Concentration of test solution (mol L−1) = 1000 × amount of test substance initial volume of test solution (mL)
= 1000 × 2.1 × 10-3(mol) 25 (mL)
= 0.084 mol L−1
An accurately weighed amount (5.1100 g) of potassium hydrogen phthalate (KHCgH404) was dissolved in distilled water (250.00 mL). This solution (25.00mL) required sodium hydroxide solution (23.50mL) to reach equivalence. What is the molarity of the sodium hydroxide solution? (Note that, in this case, 25.00mL of the standard solution was used as the titrand, whereas the test solution (NaOH) was the titrant.)
Following the sequence in Box 21.1:
- Write the equation.
⇒Equation [22.3]
NaOH(aq) + KHC8H4O4(aq) → NaKC8H4O4(aq) + H2O(I) - Determine the equivalences.Equation [22.3] shows that 1 mole of NaOH is equivalent to 1 mole of KHC8H4O4
- Calculate the number of moles of standard used.
Firstly, the concentration of the standard solution of potassium hydrogen phthalate must be calculated.
The molecular weight of KHC8H4O4 is 204.22g mol−1. Therefore 5.1100g of KHC8H4O4 is equivalent to 5.1100 (g)/204.22 (g mol−1) = 0.025 mol.
We can now calculate the concentration of the standard solution:
Concentration of standard solution (mol L−1) = 1000 × amount of KHC8H4O4 (mol) volume of standard solution (mL)
= 1000 × 0.025 (mol) 250 (mL)
= 0.100 mol L−1
The number of moles of standard used in the titration is as follows.
Number of moles
of KHC8H4O4= concentration (mol L−1) × volume (mL) 1000
= 0.100 (mol L−1) × 25.0 mL 1000
= 2.5 × 10−3 mol - Calculate the number of moles of NaOH used to reach equivalence.
As indicated in point 2 above, I mole of NaOH is equivalent to mole of KHC8H4O4. Therefore:
No. of moles of NaOH used = no. of moles of KHC8H4O4 in the titrand
= 2.5 × 10−3 mol - Determine the concentration of the NaOH solution:
Concentration of NaOH solution (mol L−1) = 1000 × amount of NaOH (mol) volume of NaOH solution used (mL)
= 1000 × 2.5 × 10−3 (mol) 23.50 (mL)
= 0.106 mol L−1