Precipitation
Inorganic ions can be separated from mixtures using organic reagents (precipitants), with which they form sparingly soluble, often coloured, compounds. The precipitants usually have high molecular weights, so a small quantity of the ion will produce a large amount of precipitate. Ideally, the precipitant should be specific for a particular ion, though this is rarely so. Examples of common precipitants and their target ions are shown in Table 20.1 and their structures are shown in Fig. 20.1.Dimethylglyoxime is only slightly soluble in water (0.40 g L−1) and it is therefore used as a 1% w/v solution in ethanol. Cupferron, the ammonium salt of N-nitroso-N-phenylhydroxylamine, is used as a 5-10%
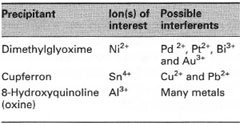 |
| Table 20.1 Common precipitants |
When precipitating a compound :
- Mix your reagents slowly, with continuous stirring, to encourage the growth of large crystals of the compound.
- Improve the precipitation process by heating your solutions: ideally, one or both solutions should be heated to just below boiling point.
- Wash your precipitate with a dilute electrolyte solution, to remove any other constituents (it is essential to remove any impurities). Choose a solution that does not interact with the precipitate, and that is volatile at the drying temperature to be used.
- Use the minimum quantity of wash solution as no precipitate is absolutely insoluble. While suitable wash solutions include dilute electrolytes, e.g. ammonium salts, ammonia solution or acids, pure water is rarely used, as it may dissolve the precipitate.
- Test your filtered wash solution for impurities using simple qualitative tests. Continue until your final washing solution contains no trace of other constituents.
- It is best to wash repeatedly with several small amounts of solution, allowing the precipitate to drain between washings.
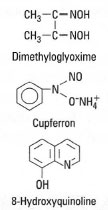 |
| Fig. 20.1 Structures of common precipitants. |




