Types of complexometric titration
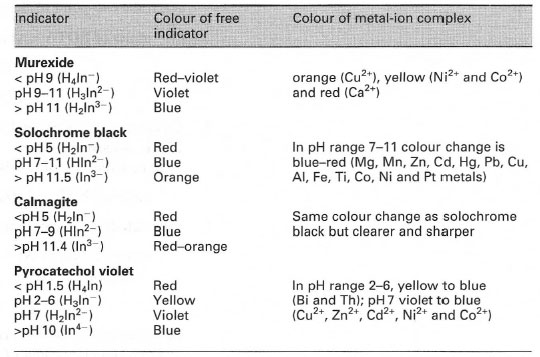
Direct titrationIn this case, the metal ion is titrated with a standard solution of EDTA. The solution containing the metal ion is buffered to an appropriate pH at which the stability constant of the metal-EDTA complex is large. The free indicator has a different colour from that of the metal-indicator complex.
Back titration
In certain circumstances a particular metal ion cannot be titrated directly. This includes situations where:
- The metal ion precipitates in the absence of EDTA.
- The metal ion reacts too slowly with EDTA.
- The metal ion forms an inert complex.
- No suitable indicator is available.
 |
| Table 23.2 Properties of selected indicators |




