Titrations
The process of adding the standard solution to the test solution is called a titration, and is carried out using a burette (see below). The point at which the reaction between the standard solution and the test substance is just complete is called the equivalence point or the theoretical (or stoichiometric) end-point. This is normally detected by a visible change, either of the standard solution itself or, more commonly, by the addition of an indicator.Standard solutions
A standard solution can be prepared by weighing out the appropriate amount of a pure reagent and making up the solution to a particular volume. The concentration of a standard solution is expressed in terms of molarity. A substance used in a primary standard should fulfil the following criteria:
- It should be obtainable in high purity (> 99.9%).
- It should remain unaltered in air during weighing (i.e. it should not be hygroscopic).
- It must not decompose when dried by heating or vacuum.
- It should be capable of being tested for impurities.
- It should be readily soluble in an appropriate solvent.
- It must react with the test substance stoichiometrically and rapidly.
Preparing a standard solution
The molarity of a solution is the concentration of the solution expressed as mol L−1. If xg of a substance of molecular weight Mr, is dissolved in ymL of distilled water, the moles of substance dissolved
| = | x | = m. | |
| Mr |
Therefore, ⇒Equation [21.1]
| molarity (mol L−1) = | m × 1000 |
| y |
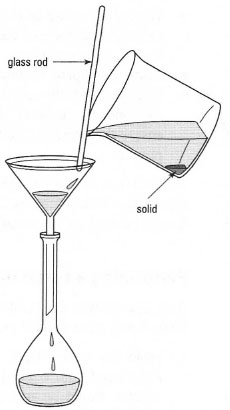
Primary standards prepared from solid compounds should be weighed out using the 'weighing by difference' method, and accurately made up to volume using a volumetric flask. Complete transfer of the substance from the weighing vessel to the volumetric flask is best achieved by inserting a funnel into the neck of the flask (Fig. 21.1). As much of the solid as possible should be transferred via the funnel. The funnel should be washed with distilled water prior to removal. Distilled water is then added to the flask, with occasional swirling to help to dissolve the solid. This is continued until the meniscus is about 1 cm below the volume mark. At this point a stopper is inserted and the flask is inverted several times to ensure the solid is completely dissolved. Finally, using a Pasteur pipette, distilled water is added up to the volume mark. The solution should be thoroughly mixed before use.
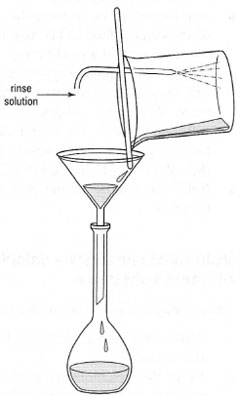
If the solid is not readily soluble in cold water it may be possible to dissolve it by stirring in warm water in a beaker. After allowing the solution to cool to room temperature, it can be transferred to the volumetric flask using a glass rod and filter funnel (Fig. 21.1) followed by several rinses of the glass rod/filter funnel. Finally, the solution is made up to the mark with distilled water.
|
|||
| Fig. 21.1 Quantitative transfer of a solid to a volumetric flask. |