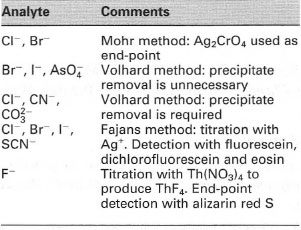
Precipitation titrations
Precipitation is the term used to describe the process whereby a substance leaves solution rapidly, forming either a crystalline solid or amorphous solid (the precipitate). In the case of a precipitation titration, this process occurs when the analyte forms a precipitate with the titrant. The most common types of precipitation titrations use silver nitrate as the titrant. They are often referred to as argentimetric titrations.Titration curves used in precipitation reactions usually use a concentrationdependent variable called the 'p function' rather than the concentration itself. The p function for a species X is defined as follows:
| ⇒Equation [25.1] | pX = − logl0 [X] |
For example, in the titration of 100mL of 0.1 mol L−1 NaC1 with 0.1 mol L−1 AgN03 the initial concentration of [Cl−] is 0.1 mol L−1, so by using eqn [25.1] the p function is 1 or pCl− = 1.
When 25mL of O.l moI L−1 AgN03 has been added, 75mL of NaCl remains in a total volume of 125mL. Therefore, the concentration of the chloride ion is given by
⇒Equation [25.2]
| [Cl−] = | 75mL × 0.1 M | = 6 × 10−2 mol L−1 | |
| 125mL |
and pCl− = 1.22. (Note that the solubility product, Ks, of AgCl is 1.1 × 10−10.
Therefore:
| ⇒Equation [25.3] | [Ag+] × [Cl−] = Ks = 1.1 × 10−10 |
| ⇒Equation [25.4] | pAg+ × pCl− = 9.96 = pAgCl |
It was found above that pCl− = 1.22, hence pAg+ = 9.96 - 1.22 = 8.74. In a similar manner, the pAg+ values can be calculated.
At the equivalence point [Ag+ ] = [Cl− ], Therefore:
| ⇒Equation [25.5] | [Ag+] × [Cl−] = √Ks = √1.1 × 10−10 = 1.05 × 10−5 |
| ⇒Equation [25.6] | pAg+ = −log(1.05 × 10−5) = 4.98 |
Beyond the equivalence point the situation changes. For 100.1mL AgN03 solution:
⇒Equation [25.7]
| [Ag+] = | 0.1 mL × 0.1 M | = 5 × 10−5 mol L−1 | |
| 200.1 mL |
or pAg+ = 4.3. Therefore,
| pCl− = pAgCl − pAg+ = 9.96 − 4.3 = 5.66 |
Values calculated in this way up to the addition of 150mL of 0.1 M AgNO3 are given in Table 25.1 and the titration curve in Fig. 25.1.
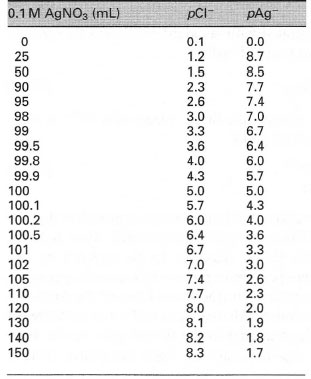 |
| Table 25. 1 Titration of 100 mL of 0.1 M NaCI with 0.1 M AgNO3. (Note that Ks, for AgCI = 1.1 × 10−10) |
End-point determination
Three techniques are commonly used to determine the end-point III precipitation titrations. They are:
- potentiometric methods;
- chemical indicator methods;
- light-scatterring methods, exemplified by turbidimetry or nephelometry.
These are:
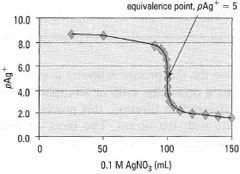 |
| Fig. 25.1 Precipitation titration curve. Initial and final slopes are drawn (see Fig. 22.3) and a parallel line is drawn such that the mid point is on the curve. This is the equivalence point. |
- Mohr titration, which involves the formation of a coloured
precipitate by reaction with the indicator. For example, in the
determination of chloride concentration with silver nitrate a small
amount of potassium chromate solution is added as an indicator.
This results in the formation of a red silver chromate (Ag2CrO4)
precipitate at the end-point:
⇒Equation [25.8] 2Ag+ + CrO42− → Ag2CrO4(s) (red)
In this case, the precipitate may form slightly after the end-point, but this error can usually be neglected. Also, the titration should be done in neutral or slighly alkaline solution (pH 6.5-9) otherwise silver chromate might not be formed. - Volhard titration, which involves the formation of a soluble coloured
compound. This approach is exemplified by the quantitative analysis of
chlorides, bromides and iodides by back titration. In this case, the halide
is titrated with silver:
⇒Equation [25.9] Ag+ + Cl− → AgCl(s)
Excess silver ions are then titrated with standard potassium thiocyanate solution in the presence of an iron (III) salt:
⇒Equation [25.10] Ag+ + SCN− → AgSCN(s)
When all the Ag+ has been reacted, the SCN- reacts with Fe3+ to form a red complex, indicating the end-point:
⇒Equation [25.11] Fe3+ + SCN− → FeSCN2+ (red)
A problem with the determination of chloride by this approach is that the end-point coloration slowly fades, as AgCl is more soluble than AgSCN. As a consequence the AgCl slowly dissolves to be replaced by the FeSCN2+. Two approaches are possible to prevent this secondary reaction from taking place. The most common method is to filter off the AgCl and titrate only the Ag+ left in solution. Alternatively, add a few millilitres of an immiscible liquid (e.g. nitrobenzene) to the titrand prior to the back titration. The nitrobenzene acts to 'coat' the AgCI precipitate, thereby isolating it from the SCN−. - Fajans titration, which involves the adsorption of a coloured indicator onto the precipitate at the end-point, resulting in a colour change. During this adsorption process a change occurs in the indicator resulting in a change of colour. The indicators used for this are often anionic dyes, e.g. fluorescein or eosin. The most common indicator for AgCl is dichlorofluorescein (Fig. 25.2) (this is greenish yellow III solution but changes colour to pink when it is ads orbed on AgCl).
|
|