Testing for anions and cations
Specific literature containing tests for the determination of anions and cations
can be found in the resources section. In general, however, the
following tips are useful when carrying out qualitative analysis:
- Always work tidily to prevent cross-contamination of samples.
- Ensure that all glassware has been cleaned thoroughly in detergent and
then rinsed twice with distilled water. Invert the test tubes to drain; never
dry the inner surface with towelling or tissue.
- Label test tubes at the start - it may prove difficult to remember what
you have done later on.
- Always test solutions with a known composition before you attempt to
analyse solutions with an unknown content. This allows you to gain the
necessary experience in solution manipulation, observation skills and the
interpretation of results.
- The colour of solutions and/or precipitates has to be interpreted from
written or oral information. Interpretation of colour can be subjective, so
you will need to gain sufficient experience using solutions of known
content to establish how a particular colour appears to you.
- Establish a protocol for recording of observations after carrying out
different tests (Fig. 19.1).
- Reagents should be added from Pasteur pipettes held with the tip just
above the mouth of the test tube. Never put Pasteur pipettes inside test
tubes as this can lead to contamination of the reagents.
- Effective mixing of the test solution and added reagents is essential. This
can be achieved by holding the test tube between the thumb and index
finger of one hand and 'flicking' the tube with the index finger of your
other hand. Alternatively, solutions can be mixed by bubbling air from a
Pasteur pipette held at the bottom of the test tube.
- Evolved gas can be drawn up into a Pasteur pipette and then bubbled
through a test solution, e.g. CO2 can be drawn into a Pasteur pipette and
then 'blown' out through lime water (Ca(OH)2 solution) giving a milkywhite
solution.
- Solutions can be tested for pH using litmus paper. Never place litmus
paper directly into the test solution. Instead, dip a glass rod into the
solution, remove, touch the wet glass rod onto the litmus paper and note
the colour. Acidic solutions change blue litmus paper to red; alkaline
solutions change red litmus paper to blue. Alternatively, universal
indicator paper can be used. In this case, the orange paper turns 'reddish'
with acidic solutions and 'bluish' with alkaline solutions. By comparing
any change in colour with a chart (supplied with the universal indicator
paper), the pH of the solution can be estimated.
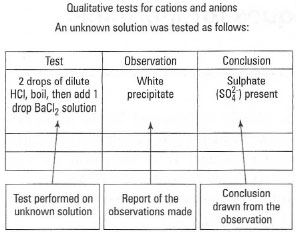 |
| Fig. 19.1 Recording your observations in qualitative analysis. |





