Chemical Energy Transfer by ATP
Chemical Energy
Transfer by ATP
We have seen that endergonic reactions are those that will not proceed spontaneously by themselves because the products require an input of free energy. However, an endergonic reaction may be driven by coupling the energy-requiring reaction with an energy-yielding reaction. ATP is the most common intermediate in coupled reactions, and because it can drive such energetically unfavorable reactions, it is of central importance in metabolic processes.
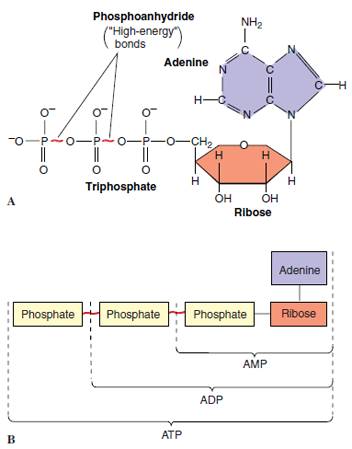
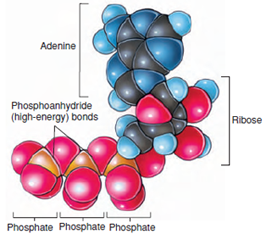
The ATP molecule consists of adenosine (the purine adenine and the 5-carbon sugar ribose) and a triphosphate group (Figures 4-6 and 4-7). Most of the free energy in ATP resides in the triphosphate group, especially in two phosphoanhydride bonds between the three phosphate groups. These two bonds are called “highenergy bonds” because a great deal of free energy in the bonds is liberated when ATP is hydrolyzed to adenosine diphosphate (ADP) and inorganic phosphate.
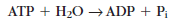
Where Pi represents inorganic phosphate (i = inorganic). The high-energy groups in ATP are designated by the “tilde” symbol ~. A high-energy phosphate bond is shown as ~P and a lowenergy bond (such as the bond linking the triphosphate group to adenosine) as —P. ATP may be symbolized as A—P~P~P and ADP as A—P~P.
The way that ATP can act to drive a
coupled reaction is shown in Figure 4-
8. A coupled reaction is really a system
involving two reactions linked by an
energy shuttle (ATP). The conversion
of substrate A to product A is endergonic
because the product contains
more free energy than the substrate.
Therefore energy must be supplied by
coupling the reaction to one that is
exergonic, the conversion of substrate
B to product B. Substrate B in this reaction
is commonly called a fuel (for
example, glucose or a lipid). Bond
energy that is released in reaction B is
transferred to ADP, which in turn is
converted to ATP. ATP now contributes
its phosphate-bond energy to reaction
A, and ADP is produced again.
The high-energy bonds of ATP are actually rather weak, unstable bonds. Because they are unstable, the energy of ATP is readily released when ATP is hydrolyzed in cellular reactions. Note that ATP is an energy-coupling agent and not a fuel. It is not a storehouse of energy set aside for some future need. Rather it is produced by one set of reactions and is almost immediately consumed by another. ATP is formed as it is needed, primarily by oxidative processes in the mitochondria. Oxygen is not consumed unless ADP and phosphate molecules are available, and these do not become available until ATP is hydrolyzed by some energyconsuming process. Metabolism is therefore mostly self-regulating.
We have seen that endergonic reactions are those that will not proceed spontaneously by themselves because the products require an input of free energy. However, an endergonic reaction may be driven by coupling the energy-requiring reaction with an energy-yielding reaction. ATP is the most common intermediate in coupled reactions, and because it can drive such energetically unfavorable reactions, it is of central importance in metabolic processes.
The ATP molecule consists of adenosine (the purine adenine and the 5-carbon sugar ribose) and a triphosphate group (Figures 4-6 and 4-7). Most of the free energy in ATP resides in the triphosphate group, especially in two phosphoanhydride bonds between the three phosphate groups. These two bonds are called “highenergy bonds” because a great deal of free energy in the bonds is liberated when ATP is hydrolyzed to adenosine diphosphate (ADP) and inorganic phosphate.
|
|
Where Pi represents inorganic phosphate (i = inorganic). The high-energy groups in ATP are designated by the “tilde” symbol ~. A high-energy phosphate bond is shown as ~P and a lowenergy bond (such as the bond linking the triphosphate group to adenosine) as —P. ATP may be symbolized as A—P~P~P and ADP as A—P~P.
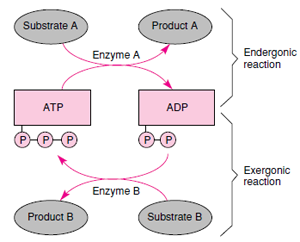 |
| Figure 4-8 A coupled reaction. The endergonic conversion of substrate A to product A will not occur spontaneously but requires an input of energy from another reaction involving a large release of energy. ATP is the intermediate through which the energy is shuttled. |
The high-energy bonds of ATP are actually rather weak, unstable bonds. Because they are unstable, the energy of ATP is readily released when ATP is hydrolyzed in cellular reactions. Note that ATP is an energy-coupling agent and not a fuel. It is not a storehouse of energy set aside for some future need. Rather it is produced by one set of reactions and is almost immediately consumed by another. ATP is formed as it is needed, primarily by oxidative processes in the mitochondria. Oxygen is not consumed unless ADP and phosphate molecules are available, and these do not become available until ATP is hydrolyzed by some energyconsuming process. Metabolism is therefore mostly self-regulating.