Enzymes and Activation Energy
The Role of
Enzymes
Enzymes and Activation Energy
For any reaction to occur, even exergonic ones that tend to proceed spontaneously, chemical bonds first must be destabilized. For example, if a reaction involves splitting a covalent bond, the atoms forming the bond must first be stretched apart to make them less stable. Some energy, termed the activation energy, must be supplied before the bond will be stressed enough to break. Only then will an overall loss of free energy and formation of reaction products occur. This requirement can be likened to the energy needed to push a cart over the crest of a hill before it will roll spontaneously down the other side, the cart liberating its potential energy as it descends.
One way to activate chemical reactants
is to raise the temperature. By
increasing the rate of molecular collisions
and pushing chemical bonds
apart, heat can impart the necessary
activation energy to make a reaction
proceed. However metabolic reactions
must occur at biologically tolerable
temperatures, temperatures too low to
allow reactions to proceed beyond
imperceptible rates. Instead, living systems
have evolved a different strategy:
they employ catalysts.
Catalysts are chemical substances that accelerate reaction rates without affecting the products of the reaction and without being altered or destroyed as a result of the reaction. A catalyst cannot make an energetically impossible reaction happen; it simply accelerates a reaction that would have proceeded at a very slow rate otherwise.
Enzymes are catalysts of the living world. The special catalytic talent of an enzyme is its power to reduce the amount of activation energy required for a reaction. In effect, an enzyme steers the reaction through one or more intermediate steps, each of which requires much less activation energy than that required for a singlestep reaction (Figure 4-3). Note that enzymes do not supply the activation energy. Instead they lower the activation energy barrier, making a reaction more likely to proceed. Enzymes affect only the reaction rate. They do not in any way alter the free energy change of a reaction, nor do they change the proportions of reactants and products in a reaction.
Enzymes and Activation Energy
For any reaction to occur, even exergonic ones that tend to proceed spontaneously, chemical bonds first must be destabilized. For example, if a reaction involves splitting a covalent bond, the atoms forming the bond must first be stretched apart to make them less stable. Some energy, termed the activation energy, must be supplied before the bond will be stressed enough to break. Only then will an overall loss of free energy and formation of reaction products occur. This requirement can be likened to the energy needed to push a cart over the crest of a hill before it will roll spontaneously down the other side, the cart liberating its potential energy as it descends.
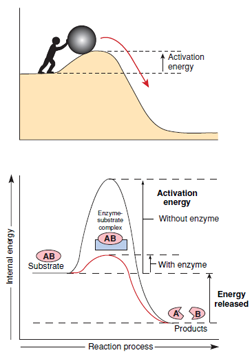 |
| Figure 4-3 Energy changes during enzyme catalysis of a substrate. The overall reaction proceeds with a net release of energy (exergonic). In the absence of an enzyme, substrate is stable because of the large amount of activation energy needed to disrupt strong chemical bonds. The enzyme reduces the energy barrier by forming a chemical intermediate with a much lower internal energy state. |
Catalysts are chemical substances that accelerate reaction rates without affecting the products of the reaction and without being altered or destroyed as a result of the reaction. A catalyst cannot make an energetically impossible reaction happen; it simply accelerates a reaction that would have proceeded at a very slow rate otherwise.
Enzymes are catalysts of the living world. The special catalytic talent of an enzyme is its power to reduce the amount of activation energy required for a reaction. In effect, an enzyme steers the reaction through one or more intermediate steps, each of which requires much less activation energy than that required for a singlestep reaction (Figure 4-3). Note that enzymes do not supply the activation energy. Instead they lower the activation energy barrier, making a reaction more likely to proceed. Enzymes affect only the reaction rate. They do not in any way alter the free energy change of a reaction, nor do they change the proportions of reactants and products in a reaction.




