Specificity of Enzymes
Specificity of Enzymes
One of the most distinctive attributes of enzymes is their high specificity. Specificity is a consequence of the exact molecular fit that is required between enzyme and substrate. Furthermore, an enzyme catalyzes only one reaction. Unlike reactions carried out in an organic chemist’s laboratory, no side reactions or by-products result. Specificity of both substrate and reaction is obviously essential to prevent a cell from being swamped with useless byproducts.
However, there is some variation
in degree of specificity. Some enzymes
catalyze the oxidation (dehydrogenation)
of only one substrate. For example,
succinic dehydrogenase catalyzes
the oxidation of succinic acid only.
Others, such as proteases (for example,
pepsin and trypsin), will act on
almost any protein, although each protease
has its particular point of attack
in the protein (Figure 4-5). Usually an
enzyme will take on one substrate
molecule at a time, catalyze its chemical
change, release the product, and
then repeat the process with another
substrate molecule. The enzyme may
repeat this process billions of times
until it is finally worn out (after a few
hours to several years) and is broken
down by scavenger enzymes in the
cell. Some enzymes undergo successive
catalytic cycles at speeds of up to
a million cycles per minute, but most
operate at slower rates.
One of the most distinctive attributes of enzymes is their high specificity. Specificity is a consequence of the exact molecular fit that is required between enzyme and substrate. Furthermore, an enzyme catalyzes only one reaction. Unlike reactions carried out in an organic chemist’s laboratory, no side reactions or by-products result. Specificity of both substrate and reaction is obviously essential to prevent a cell from being swamped with useless byproducts.
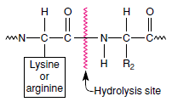 |
| Figure 4-5 High specificity of trypsin. It splits only peptide bonds adjacent to lysine or arginine. |




