Energy and the Laws of Thermodynamics
Energy and the
Laws of
Thermodynamics
The concept of energy is fundamental to all life processes. We usually express energy as the capacity to do work, that is, to bring about change. Yet energy is a somewhat abstract quantity that is difficult to define and elusive to measure. Energy cannot be seen; it can only be defined and described by how it affects matter.
Energy can exist in either of two
states: kinetic or potential. Kinetic
energy is the energy of motion. Potential energy is stored energy,
energy that is not doing work but has
the capacity to do so. Energy can be
transformed from one state to another.
Especially important for living organisms
is chemical energy, a form of
potential energy that is stored in chemical
bonds of molecules. Chemical
energy can be tapped when bonds are
rearranged to release kinetic energy.
Much of the work done by living
organisms involves the conversion of
potential energy to kinetic energy.
The conversion of one form of energy to another is governed by the two laws of thermodynamics. The first law of thermodynamics states that energy cannot be created or destroyed. It can change from one form to another, but the total amount of energy in a system remains the same. In short, energy is conserved. If we burn gasoline in an engine, we do not create new energy but merely convert the chemical energy in gasoline to another form, in this example, mechanical energy and heat. The second law of thermodynamics, introduced in the prologue to this section, concerns the transformation of energy. This fundamental law states that a closed system moves toward increasing disorder, or entropy, as energy is dissipated from the system (Figure 4- 2). Living systems, however, are open systems that not only maintain their organization but also increase it, as during the development of an animal from egg to adult.
Free Energy
To describe the energy changes that take place in chemical reactions, biochemists use the concept of free energy. Free energy is simply the energy in a system available for doing work. In a molecule, free energy equals the energy present in chemical bonds minus the energy that cannot be used. The majority of reactions in cells release free energy and are said to be exergonic (Gr. ex, out, + ergon, work). Such reactions are spontaneous and always proceed “downhill” since free energy is lost from the system. Thus:
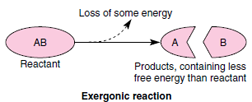
However, many important reactions in cells require the addition of free energy and are said to be endergonic (Gr. endon, within, + ergon, work). Such reactions have to be “pushed uphill” because they end up with more energy than they started with:
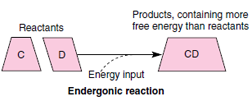
As we will see in a later section, ATP is the ubiquitous, energy-rich intermediate used by organisms to power important uphill reactions such as those required for active transport of molecules across membranes and cellular synthesis.
The concept of energy is fundamental to all life processes. We usually express energy as the capacity to do work, that is, to bring about change. Yet energy is a somewhat abstract quantity that is difficult to define and elusive to measure. Energy cannot be seen; it can only be defined and described by how it affects matter.
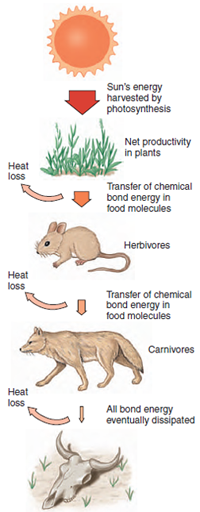 |
| Figure 4-1 Solar energy sustains virtually all life on earth. With each energy transfer, however, about 90% of the energy is lost as heat. |
The conversion of one form of energy to another is governed by the two laws of thermodynamics. The first law of thermodynamics states that energy cannot be created or destroyed. It can change from one form to another, but the total amount of energy in a system remains the same. In short, energy is conserved. If we burn gasoline in an engine, we do not create new energy but merely convert the chemical energy in gasoline to another form, in this example, mechanical energy and heat. The second law of thermodynamics, introduced in the prologue to this section, concerns the transformation of energy. This fundamental law states that a closed system moves toward increasing disorder, or entropy, as energy is dissipated from the system (Figure 4- 2). Living systems, however, are open systems that not only maintain their organization but also increase it, as during the development of an animal from egg to adult.
Free Energy
To describe the energy changes that take place in chemical reactions, biochemists use the concept of free energy. Free energy is simply the energy in a system available for doing work. In a molecule, free energy equals the energy present in chemical bonds minus the energy that cannot be used. The majority of reactions in cells release free energy and are said to be exergonic (Gr. ex, out, + ergon, work). Such reactions are spontaneous and always proceed “downhill” since free energy is lost from the system. Thus:

However, many important reactions in cells require the addition of free energy and are said to be endergonic (Gr. endon, within, + ergon, work). Such reactions have to be “pushed uphill” because they end up with more energy than they started with:

As we will see in a later section, ATP is the ubiquitous, energy-rich intermediate used by organisms to power important uphill reactions such as those required for active transport of molecules across membranes and cellular synthesis.
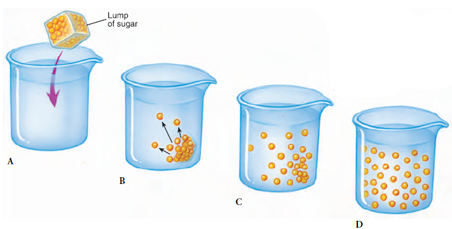 |
| Figure 4-2 Diffusion of a solute through a solution, an example of entropy. When the solute (sugar molecules) is first introduced into a solution, the system is ordered and unstable (B). Without energy to maintain this order, the solute particles become distributed into solution, reaching a state of disorder (equilibrium) (D). Entropy has increased from left diagram to right diagram. |




