How Electron Transport Is Used to Trap Chemical Bond Energy
Cellular Respiration
How Electron Transport Is Used to Trap Chemical Bond Energy
Having seen that ATP is the one common energy denominator by which all cellular machines are powered, we are in a position to ask how this energy is captured from fuel substrates. This question directs us to an important generalization: all cells obtain their chemical energy requirements from oxidation-reduction reactions. This means that in the degradation of fuel molecules, hydrogen atoms (electrons and protons) are passed from electron donors to electron acceptors with a release of energy. A portion of this energy is trapped and used to form the high-energy bonds in ATP.
Because they are so important, let
us review what we mean by oxidationreduction
(“redox”) reactions. In these
reactions there is a transfer of electrons
from an electron donor (the reducing
agent) to an electron acceptor (the oxidizing
agent). As soon as the electron
donor loses its electrons, it becomes
oxidized. As soon as the electron
acceptor accepts electrons, it becomes
reduced (Figure 4-9). In other words,
a reducing agent becomes oxidized
when it reduces another compound,
and an oxidizing agent becomes
reduced when it oxidizes another compound.
Thus for every oxidation there
must be a corresponding reduction.
In an oxidation-reduction reaction the electron donor and electron acceptor form a redox pair:
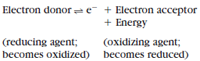
When electrons are accepted by the oxidizing agent, energy is liberated because the electrons move to a more stable position. In a cell, electrons flow through a series of carriers. Each carrier is reduced by accepting electrons and then is reoxidized by passing electrons to the next carrier in the series. By transferring electrons stepwise in this manner, energy is gradually released, and a maximum yield of ATP is realized.
How Electron Transport Is Used to Trap Chemical Bond Energy
Having seen that ATP is the one common energy denominator by which all cellular machines are powered, we are in a position to ask how this energy is captured from fuel substrates. This question directs us to an important generalization: all cells obtain their chemical energy requirements from oxidation-reduction reactions. This means that in the degradation of fuel molecules, hydrogen atoms (electrons and protons) are passed from electron donors to electron acceptors with a release of energy. A portion of this energy is trapped and used to form the high-energy bonds in ATP.
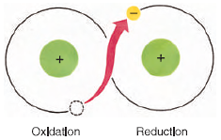 |
| Figure 4-9 A redox pair. The molecule at left is oxidized by the loss of an electron. The molecule at right is reduced by gaining an electron. |
In an oxidation-reduction reaction the electron donor and electron acceptor form a redox pair:
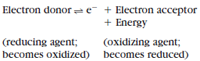
When electrons are accepted by the oxidizing agent, energy is liberated because the electrons move to a more stable position. In a cell, electrons flow through a series of carriers. Each carrier is reduced by accepting electrons and then is reoxidized by passing electrons to the next carrier in the series. By transferring electrons stepwise in this manner, energy is gradually released, and a maximum yield of ATP is realized.




