Management of Metabolism
Management
of Metabolism
The complex pattern of enzymatic
reactions that constitutes metabolism
cannot be explained entirely in terms
of physicochemical laws or chance
happenings. Although some enzymes
do indeed “flow with the tide,” the
activity of others is rigidly controlled.
In the former case, suppose the function
of an enzyme is to convert A to B.
If B is removed by conversion into
another compound, the enzyme will
tend to restore the original ratio of
B to A. Since many enzymes act
reversibly, either synthesis or degradation
may result.
For example, an
excess of an intermediate in the Krebs
cycle would result in its contribution
to glycogen synthesis; a depletion
of such a metabolite would lead
to glycogen breakdown. This automatic
compensation (equilibration) is
not, however, sufficient to explain
all that actually takes place in an
organism, as for example, what happens
at branch points in a metabolic
pathway.
Mechanisms exist for critically regulating enzymes in both quantity and activity. In bacteria, genes leading to synthesis of an enzyme are switched on or off, depending on the presence or absence of a substrate molecule. In this way the quantity of an enzyme is controlled. It is a relatively slow process.
Mechanisms that alter activity of enzymes can quickly and finely adjust metabolic pathways to changing conditions in a cell. The presence or increase in concentration of some molecules can alter the shape (conformation) of particular enzymes, thus activating or inhibiting the enzyme (Figure 4-19). For example, phosphofructokinase, which catalyzes the phosphorylation of glucose-6-phosphate to fructose-1, 6-diphosphate (Figure 4-15), is inhibited by high concentrations of ATP or citric acid. Their presence means that a sufficient amount of precursors has reached the Krebs cycle and additional glucose is not needed.
Mechanisms that alter activity of enzymes can quickly and finely adjust metabolic pathways to changing conditions in a cell. The presence or increase in concentration of some molecules can alter the shape (conformation) of particular enzymes, thus activating or inhibiting the enzyme (Figure 4-19). For example, phosphofructokinase, which catalyzes the phosphorylation of glucose-6-phosphate to fructose-1, 6-diphosphate (Figure 4-15), is inhibited by high concentrations of ATP or citric acid. Their presence means that a sufficient amount of precursors has reached the Krebs cycle and additional glucose is not needed.
As well as being subject to alteration in physical shape, many enzymes exist in both an active and an inactive form. These forms may be chemically different. Enzymes that degrade glycogen (phosphorylase) and synthesize it (synthase) are examples. Conditions that activate phosphorylase tend to inactivate synthase and vice versa.
Many cases of enzyme regulation are known, but these selected examples must suffice to illustrate the importance of enzyme regulation in the integration of metabolism.
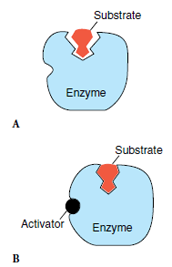 |
| Figure 4-19 Enzyme regulation. A, The active site of an enzyme may only loosely fit its substrate in the absence of an activator. B, With the regulatory site of the enzyme occupied by an activator, the enzyme binds the substrate, and the site becomes catalytically active. |
Mechanisms exist for critically regulating enzymes in both quantity and activity. In bacteria, genes leading to synthesis of an enzyme are switched on or off, depending on the presence or absence of a substrate molecule. In this way the quantity of an enzyme is controlled. It is a relatively slow process.
Mechanisms that alter activity of enzymes can quickly and finely adjust metabolic pathways to changing conditions in a cell. The presence or increase in concentration of some molecules can alter the shape (conformation) of particular enzymes, thus activating or inhibiting the enzyme (Figure 4-19). For example, phosphofructokinase, which catalyzes the phosphorylation of glucose-6-phosphate to fructose-1, 6-diphosphate (Figure 4-15), is inhibited by high concentrations of ATP or citric acid. Their presence means that a sufficient amount of precursors has reached the Krebs cycle and additional glucose is not needed.
Mechanisms that alter activity of enzymes can quickly and finely adjust metabolic pathways to changing conditions in a cell. The presence or increase in concentration of some molecules can alter the shape (conformation) of particular enzymes, thus activating or inhibiting the enzyme (Figure 4-19). For example, phosphofructokinase, which catalyzes the phosphorylation of glucose-6-phosphate to fructose-1, 6-diphosphate (Figure 4-15), is inhibited by high concentrations of ATP or citric acid. Their presence means that a sufficient amount of precursors has reached the Krebs cycle and additional glucose is not needed.
As well as being subject to alteration in physical shape, many enzymes exist in both an active and an inactive form. These forms may be chemically different. Enzymes that degrade glycogen (phosphorylase) and synthesize it (synthase) are examples. Conditions that activate phosphorylase tend to inactivate synthase and vice versa.
Many cases of enzyme regulation are known, but these selected examples must suffice to illustrate the importance of enzyme regulation in the integration of metabolism.




