Electron Transport Chain
Electron Transport Chain
Transfer of hydrogen ions and electrons from reduced NAD and FAD to the final electron acceptor, molecular oxygen, is accomplished in an elaborate electron transport chain embedded in the inner membrane of mitochondria (Figure 4-14). Each carrier molecule in the chain (labeled I to IV in Figure 4-14) is a large protein-based complex that accepts and releases electrons at lower energy levels than the carrier preceding it in the chain. As electrons pass from one carrier molecule to the next, free energy is released. Some of this energy drives the synthesis of ATP by setting up a H+ gradient across the mitochondrial membrane. At three points along the electron transport system, ATP production occurs by phosphorylation of ADP. By this means, oxidation of one NADH yields three ATP molecules. Reduced FAD from the Krebs cycle enters the electron transport chain at a lower level than NADH and so yields two ATP molecules. This method of energy capture is called oxidative phosphorylation because the formation of high-energy phosphate is coupled to oxygen consumption, and these reactions depend on the demand for ATP by other metabolic activities within the cell.
How is ATP actually generated during oxidative phosphorylation? The most widely accepted explanation is a process called chemiosmotic coupling (Figure 4-14). According to this model, as electrons contributed by NADH and FADH2 are carried down the electron transport chain, they activate proton pumping channels which pump protons (hydrogen ions) outward and into the space between the two mitochondrial membranes. This causes the proton concentration outside to rise, producing a diffusion pressure that drives the protons back into the mitochondrion through special proton channels. These channels are ATP-forming protein complexes that use the inward passage of protons to induce the formation of ATP. Exactly how proton movement is coupled to ATP synthesis is not yet understood.
Transfer of hydrogen ions and electrons from reduced NAD and FAD to the final electron acceptor, molecular oxygen, is accomplished in an elaborate electron transport chain embedded in the inner membrane of mitochondria (Figure 4-14). Each carrier molecule in the chain (labeled I to IV in Figure 4-14) is a large protein-based complex that accepts and releases electrons at lower energy levels than the carrier preceding it in the chain. As electrons pass from one carrier molecule to the next, free energy is released. Some of this energy drives the synthesis of ATP by setting up a H+ gradient across the mitochondrial membrane. At three points along the electron transport system, ATP production occurs by phosphorylation of ADP. By this means, oxidation of one NADH yields three ATP molecules. Reduced FAD from the Krebs cycle enters the electron transport chain at a lower level than NADH and so yields two ATP molecules. This method of energy capture is called oxidative phosphorylation because the formation of high-energy phosphate is coupled to oxygen consumption, and these reactions depend on the demand for ATP by other metabolic activities within the cell.
How is ATP actually generated during oxidative phosphorylation? The most widely accepted explanation is a process called chemiosmotic coupling (Figure 4-14). According to this model, as electrons contributed by NADH and FADH2 are carried down the electron transport chain, they activate proton pumping channels which pump protons (hydrogen ions) outward and into the space between the two mitochondrial membranes. This causes the proton concentration outside to rise, producing a diffusion pressure that drives the protons back into the mitochondrion through special proton channels. These channels are ATP-forming protein complexes that use the inward passage of protons to induce the formation of ATP. Exactly how proton movement is coupled to ATP synthesis is not yet understood.
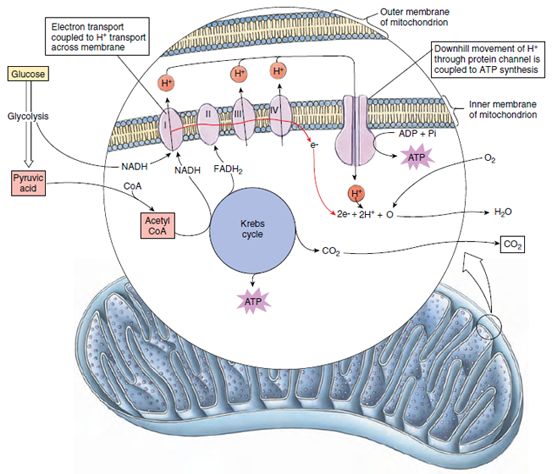 |
| Figure 4-14 Oxidative phosphorylation. Most of the ATP in living organisms is produced in the electron transport chain. Electrons removed from fuel molecules in cellular oxidations (glycolysis and the Krebs cycle) flow through the electron transport chain, the major components of which are four protein complexes (I, II, III, and IV). Electron energy is tapped by the major complexes and used to push H+ outward across the inner mitochondrial membrane. The H+ gradient created drives H+ inward through proton channels that couple H+ movement to ATP synthesis. |




